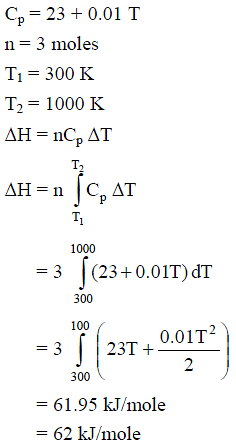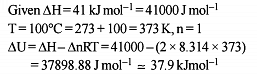Thermodynamics - 1 - JEE MCQ
30 Questions MCQ Test Chemistry for JEE Main & Advanced - Thermodynamics - 1
The pressure-volume work for an ideal gas can be calculated using the expression
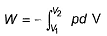
This type of work can also be calculated using the area under the curve within the specified limits. When an ideal gas is compressed, (I) reversibly or (II) irreversibly, then
In the following case
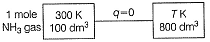 expanded in reversible adiabatic condition to make volume 8 times ( γ = 1.33). Find the Work Done and Temperature.
expanded in reversible adiabatic condition to make volume 8 times ( γ = 1.33). Find the Work Done and Temperature.
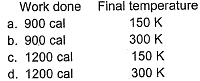
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
1.0 mole of a monoatomic ideal gas is expanded from state I to state II at 300 K.

Thus, work done is
1 mole of a diatomic gas is contained in a piston. It gains 50.0 J of heat and work is done on the surrounding by the system is -100 J. Thus,
A sample containing 1.0 mole of an ideal gas is expanded isothermally and reversibly to ten time of its original volume, in two separate experiments. The expansion is carried out 300 K and at 600 K, respectively. Choose the correct option.
During compression of a spring the work done is 10 kJ and 2 kJ escaped to the surroundings as heat. The change in internal energy, ΔU (in kJ) is :
For silver, Cp(JK–1 mol–1) = 23 + 0.01 T. If the temperature (T) of 3 moles of silver is raised from 300 K to 1000 K at 1 atm pressure, the value of ΔH will be close to -
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|