Test: Werner's theory and Valence Bond Theory - JEE MCQ
20 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Werner's theory and Valence Bond Theory
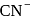 is a strong field ligand. This is due to the fact that
is a strong field ligand. This is due to the fact that
Which one of the following complexes is an outer orbital complex
Atomic nos 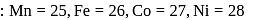
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In the complex [SbF5] 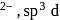 hybridisation is present Geometry of the complex is
hybridisation is present Geometry of the complex is
An anion solution gives a white ppt with 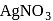 solution. The ppt. dissolves in dil. ammonia due to the formation of
solution. The ppt. dissolves in dil. ammonia due to the formation of
The spin only magnetic moment of [MnBr4]x− is 5.9BM. The geometry of the complex and x respectively are
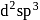 hybridisation) and is paramagnetic in nature?
hybridisation) and is paramagnetic in nature?
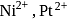 and
and  , respectively, are
, respectively, are
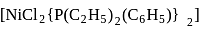 exhibits temperature dependent magnetic behaviour (paramagnetic/diamagnetic). The coordination geometries of
exhibits temperature dependent magnetic behaviour (paramagnetic/diamagnetic). The coordination geometries of 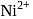 in the paramagnetic and diamagnetic states are respectively
in the paramagnetic and diamagnetic states are respectively
Which of the following complex compound(s) is/are paramagnetic and low spin?
(I) 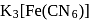
(II) 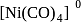
(III) 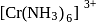
(IV) 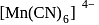
Choose the correct code:
Which of the following statement is not true for the reaction given below?
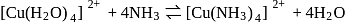
Atomic number of 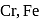 and
and 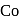 are 24, 26 and 27 respectively. Which of the following inner orbital octahedral complexes are paramagnetic?
are 24, 26 and 27 respectively. Which of the following inner orbital octahedral complexes are paramagnetic?
Which of the following statements is incorrect?
An octahedral complex of 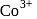 is diamagnetic. The hybridisation involved in the formation of the complex is:
is diamagnetic. The hybridisation involved in the formation of the complex is:
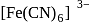 and
and  is
is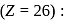
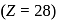 combines with a uninegative monodentate ligand to form a diamagnetic complex
combines with a uninegative monodentate ligand to form a diamagnetic complex 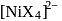 . The hybridization involved and the number of unpaired electrons present in the complex is respectively:
. The hybridization involved and the number of unpaired electrons present in the complex is respectively:
 and
and 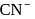 ions. It has the formula
ions. It has the formula 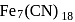 . How many
. How many 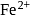 and
and  ions are there per formula unit?
ions are there per formula unit?
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|


 bond from pseudohalide to the metal and
bond from pseudohalide to the metal and 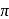 bond (from the metal to pseudohalide).
bond (from the metal to pseudohalide).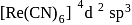 (inner)
(inner)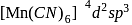 (inner)
(inner)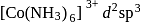 (inner)
(inner)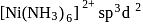 (outer)
(outer)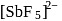 has
has 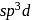 hybridization and shows square pyramidal geometry
hybridization and shows square pyramidal geometry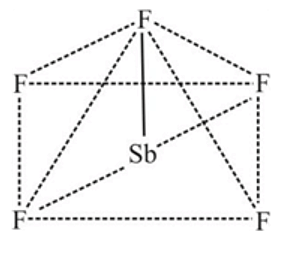

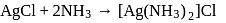

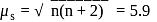 B. M.
B. M.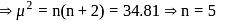
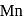 will have five unpaired electrons so
will have five unpaired electrons so 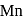 will be in +2 state with
will be in +2 state with 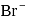 being weak- field ligands.
being weak- field ligands. for
for 

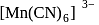 and
and 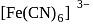 are inner orbital
are inner orbital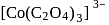 is diamagnetic in nature.
is diamagnetic in nature.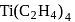 is an organometallic compound due to Ti directly attached to C- atom
is an organometallic compound due to Ti directly attached to C- atom octahedral
octahedral 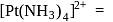 square planar
square planar 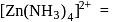 tetrahedral
tetrahedral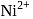 whose electronic configuration is
whose electronic configuration is  .
.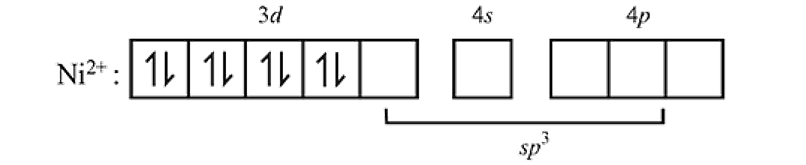
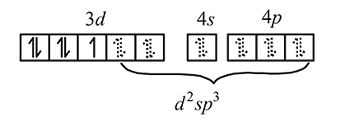
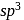 giving tetrahedral geometry. The diamagnetic state is achieved by pairing of electrons in
giving tetrahedral geometry. The diamagnetic state is achieved by pairing of electrons in 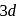 orbital.
orbital.
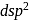 giving square planar geometry.
giving square planar geometry.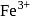 in
in 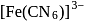
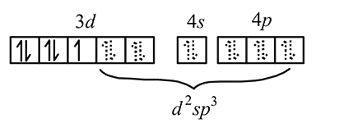
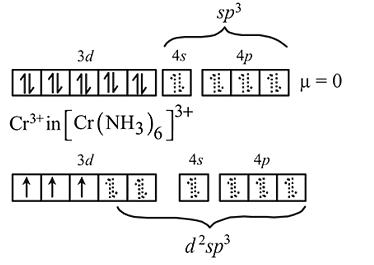
 low spin complex
low spin complex 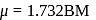 Ni in
Ni in 
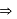 low and high spin complex is applicable for
low and high spin complex is applicable for 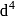 to
to 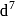 configuration
configuration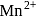 in
in 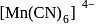
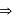 low spin complex
low spin complex 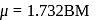
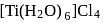
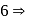 octahedral complex Ti is in
octahedral complex Ti is in  oxidations state
oxidations state  no unpaired electrons
no unpaired electrons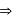 magnetic moment
magnetic moment 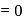 B.M.
B.M.

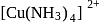 is
is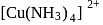 is square planar.
is square planar.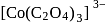 is diamagnetic as oxalate is a strong ligand causing pairing of
is diamagnetic as oxalate is a strong ligand causing pairing of  electrons in
electrons in 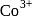 thereby leading to
thereby leading to  hybridisation.
hybridisation.
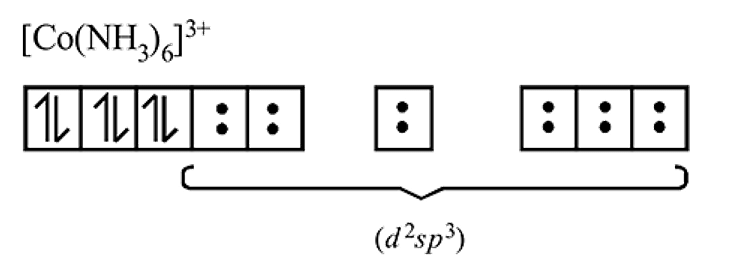
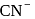 is a strong field ligand whereas
is a strong field ligand whereas 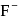 is a weak field ligand.
is a weak field ligand.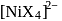 is
is 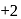 .
.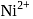 is
is 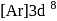 .
.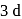 orbital empty and one
orbital empty and one 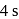 orbital empty.
orbital empty. is
is 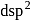 .
.











