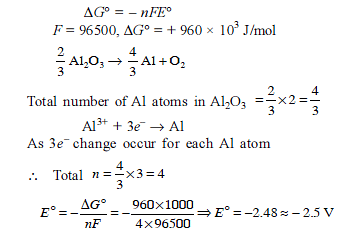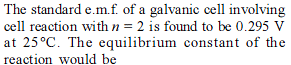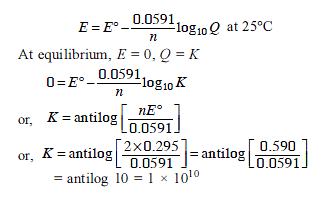Electrochemistry - 1 - JEE MCQ
30 Questions MCQ Test Chemistry for JEE Main & Advanced - Electrochemistry - 1
Which of the following reactions is possible at anode ?
[AIEEE-2002]
For a cell given below Ag | Ag+ || Cu2+ | Cu
— +
Ag+ + e- → Ag, Eº = x
Cu2+ +2e- → Cu, Eº = y
Eº cell is –
[AIEEE-2002]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The standard e.m.f. of a cell, involving one electron change is found to be 0.591 V at 25°C. The equilibrium constant of the reaction is (F = 96500 C mol-1 ; R = 8.314 JK-1mol-1)
[AIEEE-2004]
Aluminium oxide may be electrolysed at 1000ºC to furnish aluminium metal (At. Mass=27 amu ; 1 Faraday = 96,500 Coulombs). The cathode reaction is
Al3+ + 3e-→ Alº
To prepare 5.12 kg of aluminium metal by this method would require -
[AIEEE-2005]
The cell, Zn | Zn2+ (1M) | | Cu2+(1M)|Cu (Eºcell)=1.10 V), was allowed to be completely discharged at 298 K.
the relative concentration of zn2+ to 
Given EºCr3+/Cr = – 0.72 V, EºFe2+/Fe= – 0.42 V. The potential for the cell Cr |Cr3+ (0.1 M)| |Fe2+ (0.01 M) | Fe is -
[AIEEE 2008]
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|