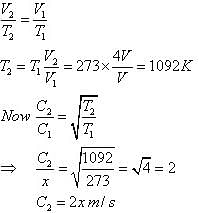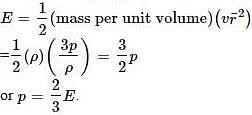Test: Kinetic Theory - 1 - NEET MCQ
10 Questions MCQ Test Physics Class 11 - Test: Kinetic Theory - 1
At absolute zero temperature may be defined as that temperature at which
Pressure exerted by an ideal gas molecule is given by the expression
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
If the r.m.s speed of oxygen at NTP is x m/s. If the gas is heated at constant pressure till its volume is four fold, what will be its final temperature and r.m.s speed?
In a mixture of ideal gases at a fixed temperature the heavier molecule has the lower average speed. This is easiest to conclude from the equation
According to kinetic theory of gases, 0K is that temperature at which for an ideal gas
The kinetic energy of one mole of an ideal gas is E=3/2 RT. Then Cρ will be
Value of gas constant, R for one mole of a gas is independent of the
The pressure exerted by an ideal gas is numerically equal to _________ of the mean kinetic energy of translation per unit volume of the gas.
The average kinetic energy of a molecule in an ideal gas is
|
97 videos|378 docs|103 tests
|