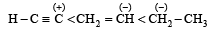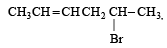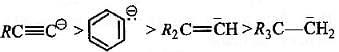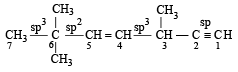31 Year NEET Previous Year Questions: Some Basic Principles & Techniques - 2 - NEET MCQ
30 Questions MCQ Test Chemistry 31 Years NEET Chapterwise Solved Papers - 31 Year NEET Previous Year Questions: Some Basic Principles & Techniques - 2
Name of the compound given below is [2003]

The correct order of reactivity towards the electrophilic substitution of the compounds aniline (I), benzene (II) and nitrobenzene (III) is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which one of the following is a free-radical substitution reaction? [2003]
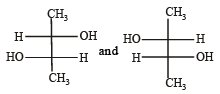
Which of the following pairs of compounds are enantiomers? [2003]
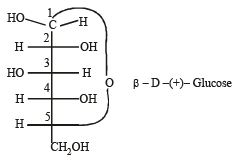
Number of chiral carbons in β - D - (+) - glucose is [2004]
Which of the following is least reactive in a nucleophilic substitution r eaction. [2004]
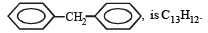
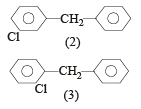
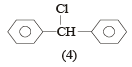
The molecular formula of diphenyl methane,
How many structural isomers are possible when one of the hydrogens is replaced by a chlorine atom?[2004]
The chirality of the compound [2005]
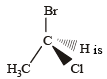
Which one of the following pairs represen ts stereoisomerism? [2005]
Which of the following undergoes nucleophilic substitution exclusively by SN1 mechanism? [2005]
Which amongst the following is the most stable carbocation? [2005]
Which one of the following compounds is most acidic? [2005]
Names of some compounds are given. Which one is not correct in IUPAC system? [2005]
The best method for the separation of naphthalene and benzoic acid from their mixture is: [2 00 5]
The general molecular formula, which represents the homologous series of alkanols is [2006]
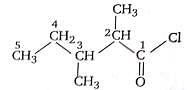
The IUPAC name of
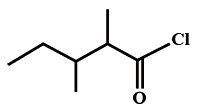 [2006]
[2006]
The correct order regarding the electronegativity of hybrid orbitals of carbon is [2006]
Which of the following is not chiral ? [2006]
Consider the following compounds. [2007]
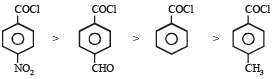
C6H5COCl
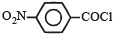
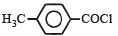
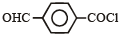
The correct decreasing order of their reactivitytowards hydrolysis is
If there is no rotation of plane polarised light by a compound in a specific solvent, though to be chiral, it may mean that [2007]
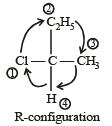
CH3 – CHCl – CH2 – CH3 has a chiral centre. which one of the following represents its R-configuration? [2007]
For (i) I–, (ii) Cl–, (iii) Br–, the increasing order of nucleophilicity would be [2007]
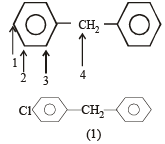
The order of decreasing reactivity towards an electrophilic reagent, for the following:
i) Benzene
ii) Toluene
iii) Chlorobenzene
iv) Phenol
Which one of the following is most reactive towards electrophilic attack ? [2008]
Base strength of : [2008]
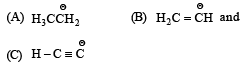
is in the order of :
How many stereoisomers does this molecule have?[2008]
CH3CH = CHCH2CHBrCH3
A strong base can abstr act an α-hydrogen from :[2008]
The stability of carbanions in the following :


is in the order of : [2008]
In the hydrocarbon
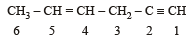
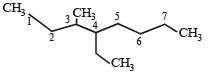
The state of hybrization of carbons 1, 3 and 5 are in the following sequence : [2008]
The state of hybridization of C2, C3, C5 and C6 of the hydrocarbon, [2009]
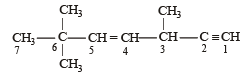
is in the following sequence:
|
29 docs|49 tests
|
|
29 docs|49 tests
|


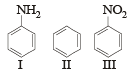
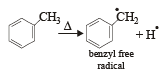
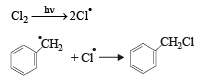

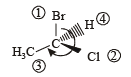
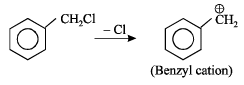
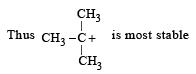
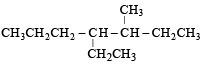

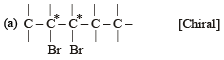
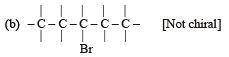
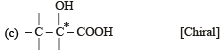
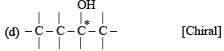
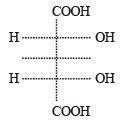 (Plane of symmetry)
(Plane of symmetry)