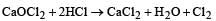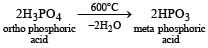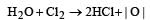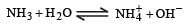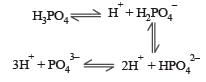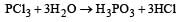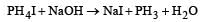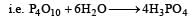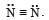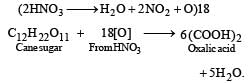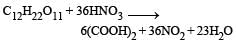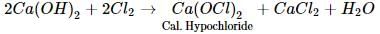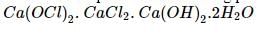31 Year NEET Previous Year Questions: The p-Block Elements - NEET MCQ
30 Questions MCQ Test Chemistry 31 Years NEET Chapterwise Solved Papers - 31 Year NEET Previous Year Questions: The p-Block Elements
Bleaching powder is obtained by the action of chlorine gas and [1988]
Hypo is used in photography to [1988]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following is a nitric acid anhydride?
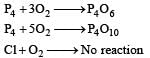
Oxygen will directly react with each of th e following elements except [1989]
Bleaching powder reacts with a few drops of dilute HCl to give [1989]
Of the following hybrides which one h as the lowest boiling point ? [1989]
Which of the following metal evolves hydrogen on reacting with cold dilute HNO3 ? [1989]
Which one of the following compounds does not exist ?[1989]
Each of the following is true about white and red phosphorus except that they [1989]
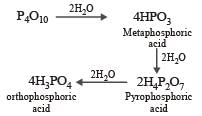
When orthophosphoric acid is heated to 600°C, the product formed is [1989]
Which one has the lowest boiling point ?[1989]
The gases respectively absor bed by alkaline pyrogallol and oil of cinnamon are [1989]
It is possible to obtain oxygen from air by fractional distillation because [1989]
Which of the following statements is not correct for nitrogen ? [1990]
The bleaching action of chlorine is due to [1991]
Which would quickly absorb oxygen ? [1991, 92]
Aqueous solution of ammonia consists of
P2O5 is heated with water to give [1991]
Basicity of orthophosphoric acid is [1991]
PCl3 reacts with water to form [1991]
PH4I + NaOH forms [1991]
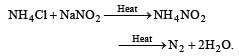
Pure nitrogen is prepared in the laboratory by heating a mixture of [1991]
Which one of the following substance is used in the laboratory for fast drying of neutral gases?[1992]
Number of electrons shared in the formation of nitrogen molecule is [1992]
Sugarcane on reaction with nitric acid gives
Nitrogen is relatively in active element because [1992]
H3PO2 is the molecular formula of an acid of phosphorus. Its name and basicity respectively are[1992]
When chlorine is passed over dry slaked lime at room temperature, the main reaction product is
In the manufacture of bromine from sea water, the mother liquor containing bromides is treated with[1992]
|
29 docs|49 tests
|
|
29 docs|49 tests
|


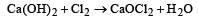
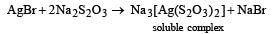
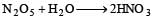
 (burns with blue light)
(burns with blue light)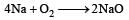 (burns with yellow light)
(burns with yellow light)