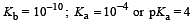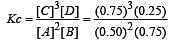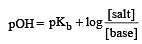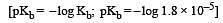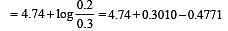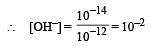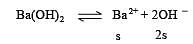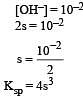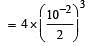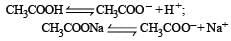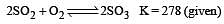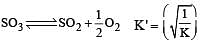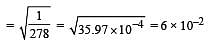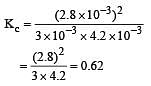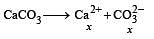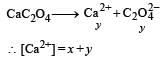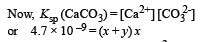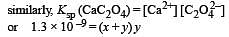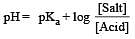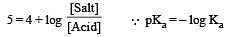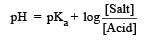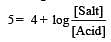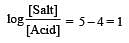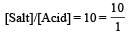31 Year NEET Previous Year Questions: Equilibrium - 1 - NEET MCQ
17 Questions MCQ Test Chemistry Class 11 - 31 Year NEET Previous Year Questions: Equilibrium - 1
In a buffer solution containing equal concentration of B– and HB, the Kb for B– is 10–10. The pH of buffer solution is : [2010]
Which one of the following molecular hydrides acts as a Lewis acid? [2010]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The reaction 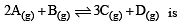 begun with the concentrations of A and B both at an initial value of 1.00 M. When equilibrium is reached, the concentration of D is measured and found to be 0.25 M. The value for the equilibrium constant for this reaction is given by the expression [2010]
begun with the concentrations of A and B both at an initial value of 1.00 M. When equilibrium is reached, the concentration of D is measured and found to be 0.25 M. The value for the equilibrium constant for this reaction is given by the expression [2010]
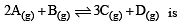 begun with the concentrations of A and B both at an initial value of 1.00 M. When equilibrium is reached, the concentration of D is measured and found to be 0.25 M. The value for the equilibrium constant for this reaction is given by the expression [2010]
begun with the concentrations of A and B both at an initial value of 1.00 M. When equilibrium is reached, the concentration of D is measured and found to be 0.25 M. The value for the equilibrium constant for this reaction is given by the expression [2010]A buffer solution is prepared in which the concentration of NH3 is 0.30M and the concentration of NH4+ is 0.20 M. If the equilibrium constant, Kb for NH3 equals 1.8 × 10–5, what is the pH of this solution ? (log 2.7 = 0.433). [2011]
Which of the following is least likely to behave as Lewis base ? [2011]
In qualitative analysis, the metals of Group I can be separated from other ions by precipitating them as chloride salts. A solution initially contains Ag+ and Pb2+ at a concentration of 0.10 M. Aqueous HCl is added to this solution until the Cl– concentration is 0.10 M. What will the concentrations of Ag+ and Pb2+ be at equilibrium?
(Ksp for AgCl = 1.8 × 10–10,
Ksp for PbCl2 = 1.7 × 10–5) [2011M]
pH of a saturated solution of Ba(OH)2 is 12. The value of solubility product (KSP)of Ba(OH)2 is : [2012]
Equimolar solutions of the following substances were prepared separately. Which one of these will record the highest pH value ? [2012]
Buffer solutions have con stant acidity and alkalinity because [2012]
Given that the equilibrium constant for the reaction 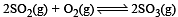 has a value of 278 at a particular temperature. What is the value of the equilibrium constant for the following reaction at the same temperature ?
has a value of 278 at a particular temperature. What is the value of the equilibrium constant for the following reaction at the same temperature ?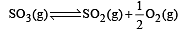 [2012 M]
[2012 M]
Given the reaction between 2 gases represented by A2 and B2 to give the compound AB(g).
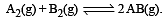
At equilibrium, the concentration
of A2 = 3.0 × 10–3 M
of B2= 4.2 × 10–3 M
of AB = 2.8 × 10–3 M
lf the reaction takes place in a sealed vessel at 527°C, then the value of KC will be : [2012 M]
Identify the correct order of solubility in aqueous medium: [NEET 2013]
Which of these is least likely to act as Lewis base? [NEET 2013]
The values of Ksp of CaCO3 and CaC2O4 are 4.7 × 10–9 and 1.3 × 10–9 respectively at 25°C. If the mixture of these two is washed with water, what is the concentration of Ca2+ ions in water? [NEET Kar. 2013]
At 100°C the Kw of water is 55 times its value at 25°C. What will be the pH of neutral solution? (log 55 = 1.74) [NEET Kar. 2013]
Th e dissociation constan t of a weak acid is 1 × 10– 4. In order to prepare a buffer solution with a pH = 5 the [Salt]/[Acid] ratio should be [NEET Kar. 2013]
Accumulation of lactic acid (HC3H5O3), a monobasic acid in tissues leads to pain and a feeling of fatigue. In a 0.10 M aqueous solution, lactic acid is 3.7% dissociated. The value of dissociation constant, Ka, for this acid will be: [NEET Kar. 2013]
|
129 videos|238 docs|88 tests
|