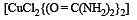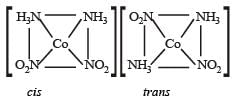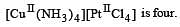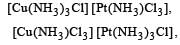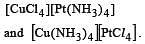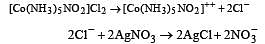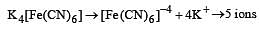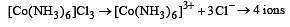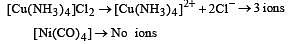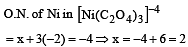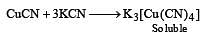32 Year NEET Previous Year Questions: Coordination Compounds - 1 - NEET MCQ
30 Questions MCQ Test - 32 Year NEET Previous Year Questions: Coordination Compounds - 1
An example of double salt is [1989]
The complex ion [Co(NH3)6]3+ is formed by sp3d2 hybridisation. Hence the ion should possess
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
K3[Al(C2O4)3] is called [1994]
Among the following complexes, optical activity is possible in [1994]
Which of the following is common donor atom in ligands? [1995]
Among the following, the compound that is both paramagnetic and coloured, is [1996]
Which of the following may be considered to be an organometallic compound? [1996]
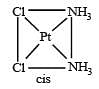
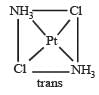
The number of geometrical isomers for [Pt (NH3)2 Cl2] is
The formula for the complex, dichlorobis (urea) copper (II) is [1997]
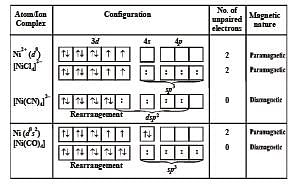
Which of the following statements is correct ? (Atomic number of Ni = 28) [1997]
The number of geometrical isomers of the complex [Co(NO2)3 (NH3)3] is [1997]
The total number of possible isomers for the complex compound [CuII (NH3)4] [PtII Cl4] [1998]
IUPAC name of [Pt(NH3)3 (Br) (NO2) Cl] Cl is [1998]
A co-ordination complex compound of cobalt has the molecular formula containing five ammonia molecules, one nitro group and two chlorine atoms for one cobalt atom. One mole of this compound produces three mole ions in an aqueous solution. On reacting this solution with excess of AgNO3 solution, we get two moles of AgCl precipitate. The ionic formula for this complex would be [1998]
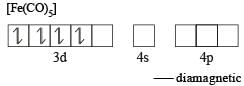
In which of the following compounds does iron exhibit zero oxidation state? [1999]
Which one of the following will show paramagnetism corresponding to 2 unpaired electrons?(Atomic numbers : Ni = 28, Fe = 26) [1999]
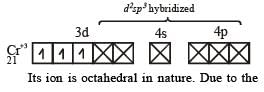
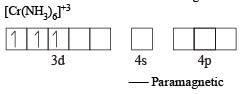
The number of unpaired electrons in the complex [Cr(NH3)6]Br3 is (Atomic number Cr = 24)
Which one of the following complexes will have four different isomers ? [2000]
Which of the following will give maximum number of isomers? [2001]
Which of the following will exhibit maximum ionic conductivity? [2001]
Which statement is incorrect? [2001]
Which of the following organometallic compound is σ and π bonded? [2001]
Oxidation number of Ni in [Ni(C2O4)3]4– is [2001]
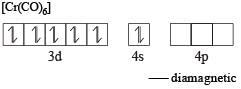
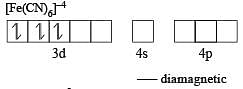
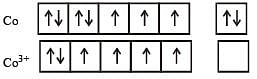
Atomic number of Cr and Fe are respectively 24 and 26, which of the following is paramagnetic? [2002]
The hypothetical complex chlorodiaquotriammine cobalt (III) chloride can be represented as [2002]
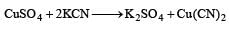
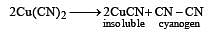
CuSO4 when reacts with KCN forms CuCN, which is insoluble in water. It is soluble in excess of KCN due to formation of the following complex [2002]
Which one of the following octahedral complexes will not show geometric isomerism? ( A and B are monodentate ligands) [2003]
According to IUPAC nomenclature sodium nitroprusside is named as [2003]
The number of unpaired electrons in the complex ion [CoF6]3– is (Atomic no.: Co = 27) [2003]
Among the following, which is not the π-bonded organometallic compound? [2003]