Case Based Questions Test: Dual Nature of Radiation & Matter - NEET MCQ
15 Questions MCQ Test Topic-wise MCQ Tests for NEET - Case Based Questions Test: Dual Nature of Radiation & Matter
Read the following text and answer the following questions on the basis of the same:
Photocell: A photocell is a technological application of the photoelectric effect. It is a device whose electrical properties are affected by light. It is also sometimes called an electric eye. A photocell consists of a semi-cylindrical photo-sensitive metal plate C (emitter) and a wire loop A (collector) supported in an evacuated glass or quartz bulb. It is connected to the external circuit having a high-tension battery B and micro ammeter (µA) as shown in the Figure.
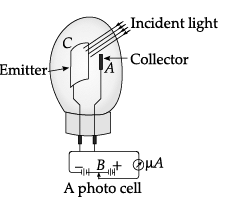
Sometimes, instead of the plate C, a thin layer of photosensitive material is pasted on the inside of the bulb. A part of the bulb is left clean for the light to enter it. When light of suitable wavelength falls on the emitter C, photoelectrons are emitted. These photoelectrons are drawn to the collector A. Photocurrent of the order of a few microampere can be normally obtained from a photo cell. A photocell converts a change in intensity of illumination into a change in photocurrent. This current can be used to operate control systems and in light measuring devices.
Q. Photosensitive material should be connected to

Read the following text and answer the following questions on the basis of the same:
Photocell: A photocell is a technological application of the photoelectric effect. It is a device whose electrical properties are affected by light. It is also sometimes called an electric eye. A photocell consists of a semi-cylindrical photo-sensitive metal plate C (emitter) and a wire loop A (collector) supported in an evacuated glass or quartz bulb. It is connected to the external circuit having a high-tension battery B and micro ammeter (µA) as shown in the Figure.

Sometimes, instead of the plate C, a thin layer of photosensitive material is pasted on the inside of the bulb. A part of the bulb is left clean for the light to enter it. When light of suitable wavelength falls on the emitter C, photoelectrons are emitted. These photoelectrons are drawn to the collector A. Photocurrent of the order of a few microampere can be normally obtained from a photo cell. A photocell converts a change in intensity of illumination into a change in photocurrent. This current can be used to operate control systems and in light measuring devices.
Q. The photocurrent generated is in the order of

| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Read the following text and answer the following questions on the basis of the same:
Photocell: A photocell is a technological application of the photoelectric effect. It is a device whose electrical properties are affected by light. It is also sometimes called an electric eye. A photocell consists of a semi-cylindrical photo-sensitive metal plate C (emitter) and a wire loop A (collector) supported in an evacuated glass or quartz bulb. It is connected to the external circuit having a high-tension battery B and micro ammeter (µA) as shown in the Figure.

Sometimes, instead of the plate C, a thin layer of photosensitive material is pasted on the inside of the bulb. A part of the bulb is left clean for the light to enter it. When light of suitable wavelength falls on the emitter C, photoelectrons are emitted. These photoelectrons are drawn to the collector A. Photocurrent of the order of a few microampere can be normally obtained from a photo cell. A photocell converts a change in intensity of illumination into a change in photocurrent. This current can be used to operate control systems and in light measuring devices.
Q. Photocell is an application of

Read the following text and answer the following questions on the basis of the same:
Photocell: A photocell is a technological application of the photoelectric effect. It is a device whose electrical properties are affected by light. It is also sometimes called an electric eye. A photocell consists of a semi-cylindrical photo-sensitive metal plate C (emitter) and a wire loop A (collector) supported in an evacuated glass or quartz bulb. It is connected to the external circuit having a high-tension battery B and micro ammeter (µA) as shown in the Figure.
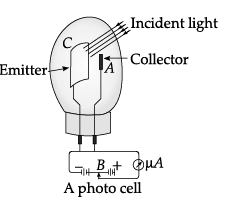
Sometimes, instead of the plate C, a thin layer of photosensitive material is pasted on the inside of the bulb. A part of the bulb is left clean for the light to enter it. When light of suitable wavelength falls on the emitter C, photoelectrons are emitted. These photoelectrons are drawn to the collector A. Photocurrent of the order of a few microampere can be normally obtained from a photo cell. A photocell converts a change in intensity of illumination into a change in photocurrent. This current can be used to operate control systems and in light measuring devices.
Q. Which of the following statement is true?
Read the following text and answer the following questions on the basis of the same:
Photocell: A photocell is a technological application of the photoelectric effect. It is a device whose electrical properties are affected by light. It is also sometimes called an electric eye. A photocell consists of a semi-cylindrical photo-sensitive metal plate C (emitter) and a wire loop A (collector) supported in an evacuated glass or quartz bulb. It is connected to the external circuit having a high-tension battery B and micro ammeter (µA) as shown in the Figure.
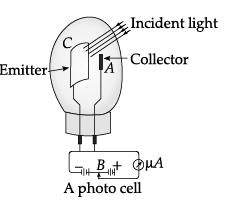
Sometimes, instead of the plate C, a thin layer of photosensitive material is pasted on the inside of the bulb. A part of the bulb is left clean for the light to enter it. When light of suitable wavelength falls on the emitter C, photoelectrons are emitted. These photoelectrons are drawn to the collector A. Photocurrent of the order of a few microampere can be normally obtained from a photo cell. A photocell converts a change in intensity of illumination into a change in photocurrent. This current can be used to operate control systems and in light measuring devices.
Q. A photocell converts a change in ___ of incident light into a change in ____ _
Read the following text and answer the following questions on the basis of the same:
Electron Microscope Electron microscopes use electrons to illuminate a sample. In Transmission Electron Microscopy (TEM), electrons pass through the sample and illuminate film or a digital camera.
Resolution in microscopy is limited to about half of the wavelength of the illumination source used to image the sample. Using visible light the best resolution that can be achieved by microscopes is about ~200 nm. Louis de Broglie showed that every particle or matter propagates like a wave. The wavelength of propagating electrons at a given accelerating voltage can be determined by

Thus, the wavelength of electrons is calculated to be 3.88 pm when the microscope is operated at 100 keV, 2. 74 pm at 200 keV and 2.24 pm at 300 keV. However, because the velocities of electrons in an electron microscope reach about 70% the speed of light with an accelerating voltage of 200 keV, there are relativistic effects on these electrons. Due to this effect, the wavelength at 100 keV, 200 keV and 300 keV in electron microscopes is 3.70 pm, 2.51 pm and 1.96 pm, respectively.
Anyhow, the wavelength of electrons is much smaller than that of photons (2.5 pm at 200 keV). Thus if electron wave is used to illuminate the sample, the resolution of an electron microscope theoretically becomes unlimited. Practically, the resolution is limited to ~0.1 nm due to the objective lens system in electron microscopes. Thus, electron microscopy can resolve subcellular structures that could not be visualized using standard fluorescences microscopy.
Q. Who showed that electron also propagates like a wave?
Read the following text and answer the following questions on the basis of the same:
Electron Microscope Electron microscopes use electrons to illuminate a sample. In Transmission Electron Microscopy (TEM), electrons pass through the sample and illuminate film or a digital camera.
Resolution in microscopy is limited to about half of the wavelength of the illumination source used to image the sample. Using visible light the best resolution that can be achieved by microscopes is about ~200 nm. Louis de Broglie showed that every particle or matter propagates like a wave. The wavelength of propagating electrons at a given accelerating voltage can be determined by

Thus, the wavelength of electrons is calculated to be 3.88 pm when the microscope is operated at 100 keV, 2. 74 pm at 200 keV and 2.24 pm at 300 keV. However, because the velocities of electrons in an electron microscope reach about 70% the speed of light with an accelerating voltage of 200 keV, there are relativistic effects on these electrons. Due to this effect, the wavelength at 100 keV, 200 keV and 300 keV in electron microscopes is 3.70 pm, 2.51 pm and 1.96 pm, respectively.
Anyhow, the wavelength of electrons is much smaller than that of photons (2.5 pm at 200 keV). Thus if electron wave is used to illuminate the sample, the resolution of an electron microscope theoretically becomes unlimited. Practically, the resolution is limited to ~0.1 nm due to the objective lens system in electron microscopes. Thus, electron microscopy can resolve subcellular structures that could not be visualized using standard fluorescences microscopy.
Q. As the accelerating voltage increases, the wavelength of electron as wave
Read the following text and answer the following questions on the basis of the same:
Electron Microscope Electron microscopes use electrons to illuminate a sample. In Transmission Electron Microscopy (TEM), electrons pass through the sample and illuminate film or a digital camera.
Resolution in microscopy is limited to about half of the wavelength of the illumination source used to image the sample. Using visible light the best resolution that can be achieved by microscopes is about ~200 nm. Louis de Broglie showed that every particle or matter propagates like a wave. The wavelength of propagating electrons at a given accelerating voltage can be determined by

Thus, the wavelength of electrons is calculated to be 3.88 pm when the microscope is operated at 100 keV, 2. 74 pm at 200 keV and 2.24 pm at 300 keV. However, because the velocities of electrons in an electron microscope reach about 70% the speed of light with an accelerating voltage of 200 keV, there are relativistic effects on these electrons. Due to this effect, the wavelength at 100 keV, 200 keV and 300 keV in electron microscopes is 3.70 pm, 2.51 pm and 1.96 pm, respectively.
Anyhow, the wavelength of electrons is much smaller than that of photons (2.5 pm at 200 keV). Thus if electron wave is used to illuminate the sample, the resolution of an electron microscope theoretically becomes unlimited. Practically, the resolution is limited to ~0.1 nm due to the objective lens system in electron microscopes. Thus, electron microscopy can resolve subcellular structures that could not be visualized using standard fluorescences microscopy.
Q. In electron microscope, electron is used:
Read the following text and answer the following questions on the basis of the same:
Electron Microscope Electron microscopes use electrons to illuminate a sample. In Transmission Electron Microscopy (TEM), electrons pass through the sample and illuminate film or a digital camera.
Resolution in microscopy is limited to about half of the wavelength of the illumination source used to image the sample. Using visible light the best resolution that can be achieved by microscopes is about ~200 nm. Louis de Broglie showed that every particle or matter propagates like a wave. The wavelength of propagating electrons at a given accelerating voltage can be determined by

Thus, the wavelength of electrons is calculated to be 3.88 pm when the microscope is operated at 100 keV, 2. 74 pm at 200 keV and 2.24 pm at 300 keV. However, because the velocities of electrons in an electron microscope reach about 70% the speed of light with an accelerating voltage of 200 keV, there are relativistic effects on these electrons. Due to this effect, the wavelength at 100 keV, 200 keV and 300 keV in electron microscopes is 3.70 pm, 2.51 pm and 1.96 pm, respectively.
Anyhow, the wavelength of electrons is much smaller than that of photons (2.5 pm at 200 keV). Thus if electron wave is used to illuminate the sample, the resolution of an electron microscope theoretically becomes unlimited. Practically, the resolution is limited to ~0.1 nm due to the objective lens system in electron microscopes. Thus, electron microscopy can resolve subcellular structures that could not be visualized using standard fluorescences microscopy.
Q. Why electron as wave is used in electron microscope to illuminate the sample?
Read the following text and answer the following questions on the basis of the same:
Electron Microscope Electron microscopes use electrons to illuminate a sample. In Transmission Electron Microscopy (TEM), electrons pass through the sample and illuminate film or a digital camera.
Resolution in microscopy is limited to about half of the wavelength of the illumination source used to image the sample. Using visible light the best resolution that can be achieved by microscopes is about ~200 nm. Louis de Broglie showed that every particle or matter propagates like a wave. The wavelength of propagating electrons at a given accelerating voltage can be determined by

Thus, the wavelength of electrons is calculated to be 3.88 pm when the microscope is operated at 100 keV, 2. 74 pm at 200 keV and 2.24 pm at 300 keV. However, because the velocities of electrons in an electron microscope reach about 70% the speed of light with an accelerating voltage of 200 keV, there are relativistic effects on these electrons. Due to this effect, the wavelength at 100 keV, 200 keV and 300 keV in electron microscopes is 3.70 pm, 2.51 pm and 1.96 pm, respectively.
Anyhow, the wavelength of electrons is much smaller than that of photons (2.5 pm at 200 keV). Thus if electron wave is used to illuminate the sample, the resolution of an electron microscope theoretically becomes unlimited. Practically, the resolution is limited to ~0.1 nm due to the objective lens system in electron microscopes. Thus, electron microscopy can resolve subcellular structures that could not be visualized using standard fluorescence microscopy.
Q. Wavelength of electron as wave at accelerating voltage 200 keV is
According to wave theory of light, the light of any frequency can emit electrons from metallic surface provided the intensity of light be sufficient to provide necessary energy for emission of electrons, but according to experimental observations, the light of frequency less than threshold frequency can not emit electrons; whatever be the intensity of incident light. Einstein also proposed that electromagnetic radiation is quantised.
If photoelectrons are ejected from a surface when light of wavelength λ1 = 550 nm is incident on it. The stopping potential for such electrons is Vs =0.19 . Suppose the radiation of wavelength λ2 = 190 nm is incident on the surface.
Q. In photoelectric effect, electrons are ejected from metals, if the incident light has a certain minimum
According to wave theory of light, the light of any frequency can emit electrons from metallic surface provided the intensity of light be sufficient to provide necessary energy for emission of electrons, but according to experimental observations, the light of frequency less than threshold frequency can not emit electrons; whatever be the intensity of incident light. Einstein also proposed that electromagnetic radiation is quantised.
If photoelectrons are ejected from a surface when light of wavelength λ1 = 550 nm is incident on it. The stopping potential for such electrons is Vs =0.19 . Suppose the radiation of wavelength λ2 = 190 nm is incident on the surface.
Q. Calculate the work function of the surface.
According to wave theory of light, the light of any frequency can emit electrons from metallic surface provided the intensity of light be sufficient to provide necessary energy for emission of electrons, but according to experimental observations, the light of frequency less than threshold frequency can not emit electrons; whatever be the intensity of incident light. Einstein also proposed that electromagnetic radiation is quantised.
If photoelectrons are ejected from a surface when light of wavelength λ1 = 550 nm is incident on it. The stopping potential for such electrons is Vs =0.19 . Suppose the radiation of wavelength λ2 = 190 nm is incident on the surface.
Q. Photoelectric effect supports quantum nature of light because
(i) there is a minimum frequency of light below which no photoelectrons are emitted.
(ii) the maximum K.E. of photoelectric depends only on the frequency of light and not on its intensity.
(iii) even when the metal surface is faintly illuminated, the photo electrons leave the surface immediately.
(iv) electric charge of the photoelectrons is quantized.
According to wave theory of light, the light of any frequency can emit electrons from metallic surface provided the intensity of light be sufficient to provide necessary energy for emission of electrons, but according to experimental observations, the light of frequency less than threshold frequency can not emit electrons; whatever be the intensity of incident light. Einstein also proposed that electromagnetic radiation is quantised.
If photoelectrons are ejected from a surface when light of wavelength λ1 = 550 nm is incident on it. The stopping potential for such electrons is Vs =0.19 . Suppose the radiation of wavelength λ2 = 190 nm is incident on the surface.
Q. Calculate the stopping potential Vs2 of the surface.
According to wave theory of light, the light of any frequency can emit electrons from metallic surface provided the intensity of light be sufficient to provide necessary energy for emission of electrons, but according to experimental observations, the light of frequency less than threshold frequency can not emit electrons; whatever be the intensity of incident light. Einstein also proposed that electromagnetic radiation is quantised.
If photoelectrons are ejected from a surface when light of wavelength λ1 = 550 nm is incident on it. The stopping potential for such electrons is Vs =0.19 . Suppose the radiation of wavelength λ2 = 190 nm is incident on the surface.
Q. Calculate the threshold frequency for the surface.
|
9 docs|1272 tests
|




 - (1.6 × 10−19)(0.19) = 3.31 × 10−19J
- (1.6 × 10−19)(0.19) = 3.31 × 10−19J = 0.5 × 1015 = 500 × 1012Hz
= 0.5 × 1015 = 500 × 1012Hz














