NEET Exam > NEET Tests > Chemistry Class 11 > Test: Atomic & Molecular Mass (NCERT) - NEET MCQ
Test: Atomic & Molecular Mass (NCERT) - NEET MCQ
Test Description
5 Questions MCQ Test Chemistry Class 11 - Test: Atomic & Molecular Mass (NCERT)
Test: Atomic & Molecular Mass (NCERT) for NEET 2024 is part of Chemistry Class 11 preparation. The Test: Atomic & Molecular Mass (NCERT) questions and answers have been
prepared according to the NEET exam syllabus.The Test: Atomic & Molecular Mass (NCERT) MCQs are made for NEET 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Atomic & Molecular Mass (NCERT) below.
Solutions of Test: Atomic & Molecular Mass (NCERT) questions in English are available as part of our Chemistry Class 11 for NEET & Test: Atomic & Molecular Mass (NCERT) solutions in
Hindi for Chemistry Class 11 course. Download more important topics, notes, lectures and mock
test series for NEET Exam by signing up for free. Attempt Test: Atomic & Molecular Mass (NCERT) | 5 questions in 5 minutes | Mock test for NEET preparation | Free important questions MCQ to study Chemistry Class 11 for NEET Exam | Download free PDF with solutions
Test: Atomic & Molecular Mass (NCERT) - Question 1
The reference standard used for defining atomic mass is
Detailed Solution for Test: Atomic & Molecular Mass (NCERT) - Question 1
Test: Atomic & Molecular Mass (NCERT) - Question 2
Atomic masses of elements are usually fractional because
Detailed Solution for Test: Atomic & Molecular Mass (NCERT) - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Atomic & Molecular Mass (NCERT) - Question 3
Oxygen occurs in nature as a mixture of isotopes 16O, 17O, and 18O having atomic masses of 15.995 u, 16.999 u and 17.999 u and relative abundance of 99.763%, 0.037% and 0.200% respectively. What is the average atomic mass of oxygen?
Detailed Solution for Test: Atomic & Molecular Mass (NCERT) - Question 3
Test: Atomic & Molecular Mass (NCERT) - Question 4
Calculate the molecular mass of sucrose(C12H22O11) molecule?
Detailed Solution for Test: Atomic & Molecular Mass (NCERT) - Question 4
Test: Atomic & Molecular Mass (NCERT) - Question 5
What mass of hydrochloric acid will contain the same number of hydrogen atoms as are present in 16 g of methane?
Detailed Solution for Test: Atomic & Molecular Mass (NCERT) - Question 5
|
129 videos|238 docs|88 tests
|
Information about Test: Atomic & Molecular Mass (NCERT) Page
In this test you can find the Exam questions for Test: Atomic & Molecular Mass (NCERT) solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Atomic & Molecular Mass (NCERT), EduRev gives you an ample number of Online tests for practice


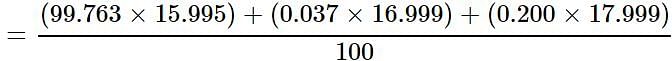 = 15.999 u
= 15.999 u















