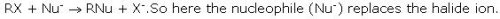NEET Exam > NEET Tests > Test: Chemical Properties - NEET MCQ
Test: Chemical Properties - NEET MCQ
Test Description
10 Questions MCQ Test - Test: Chemical Properties
Test: Chemical Properties for NEET 2025 is part of NEET preparation. The Test: Chemical Properties questions and answers have been prepared
according to the NEET exam syllabus.The Test: Chemical Properties MCQs are made for NEET 2025 Exam.
Find important definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Chemical Properties below.
Solutions of Test: Chemical Properties questions in English are available as part of our course for NEET & Test: Chemical Properties solutions in
Hindi for NEET course.
Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free. Attempt Test: Chemical Properties | 10 questions in 15 minutes | Mock test for NEET preparation | Free important questions MCQ to study for NEET Exam | Download free PDF with solutions
Test: Chemical Properties - Question 1
In dehydrohalogenation reactions the preferred product is that alkene which has the greatest number of alkyl groups attached to the doubly bonded carbon atoms. This rule is known as:
Detailed Solution for Test: Chemical Properties - Question 1
Test: Chemical Properties - Question 2
Which one of the following is an organometallic compound?
Detailed Solution for Test: Chemical Properties - Question 2
Test: Chemical Properties - Question 3
Which one of the following compounds has zero dipole moment?
Detailed Solution for Test: Chemical Properties - Question 3
Detailed Solution for Test: Chemical Properties - Question 4
Test: Chemical Properties - Question 5
Which of the following compound does have chiral carbon atom?
Detailed Solution for Test: Chemical Properties - Question 5
Detailed Solution for Test: Chemical Properties - Question 6
Test: Chemical Properties - Question 7
In the nucleophilic substitution reaction in alkyl halides (R-X) , the nucleophile replaces:
Detailed Solution for Test: Chemical Properties - Question 7
Test: Chemical Properties - Question 8
Reactivity of alkyl halides towards SN1 nucleophilic substitution reaction is:
Detailed Solution for Test: Chemical Properties - Question 8
Test: Chemical Properties - Question 9
The inversion of configuration of optically active alkyl halides occurs in
Detailed Solution for Test: Chemical Properties - Question 9
Test: Chemical Properties - Question 10
What is formed when ethyl magnesium bromide reacts with water?
Detailed Solution for Test: Chemical Properties - Question 10
Information about Test: Chemical Properties Page
In this test you can find the Exam questions for Test: Chemical Properties solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Chemical Properties, EduRev gives you an ample number of Online tests for practice
Download as PDF