Test: Colligative Properties (Elevation in Boiling Point) - NEET MCQ
20 Questions MCQ Test Topic-wise MCQ Tests for NEET - Test: Colligative Properties (Elevation in Boiling Point)
Only One Option Correct Type
This section contains 13 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
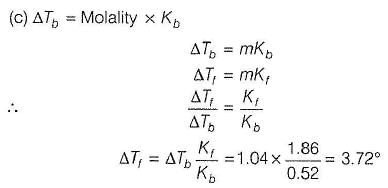
Elevation in boiling point of an aqueous solution of a non-electrolyte solute is 1.04°. What is the depression in freezing point of this solution?
Kf (H20) = 0.52° mol-1 kg
Kf (H20) = 1.86° mol-1 kg
This section contains 13 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct
12.8 g of naphthalene (C10H8) is dissolved in 92 g toluene (C7H8). Boiling point of this solution is
(boiling point of toluene = 110°Cand mole fraction boiling point elevation constant of toluene is 36.1 K)
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Elevation in boiling point of a molar (1 M) glucose solution (d = 1.2 g mL-1) is
For a dilute solution containing 2.5 g of a non-volatile non-electrolyte solute in 100 g water, the elevation in boiling point at 1 atm pressure is 2°C. Assuming concentration of solute is much lower than the concentration of solvent, the vapour pressure (mm of Hg) of the solution is (Take, Kb = 0.76 K mol-1 kg).
[IITJEE2012]
Relative lowering of vapour pressure of a non-volatile solute X in water is 0.05. If Kb(H20) = 0.52° mol-1 kg-1.
(ΔTb) = elevation in boiling point assuming a dilute solution
(ATb)' = elevation in boiling point assuming a solution of moderate concentration then, {ΔTb) - {ΔTb)' =
6% aqueous solution of urea (by mass of solvent) has elevation in boiling point xt. 18% aqueous solution of glucose (by mass of solvent) has elevation in boiling point x2. If these two solutions are mixed then, elevation in boiling point is x3. Then,
AB is a sparingly soluble salt in aqueous solution with Ksp =1x10-8. If Kb(H20 ) = 0.52° mol-1 kg then elevation in boiling point of saturated solution is
Elevation in boiling point of a solution of non-electrolyte in non-polar solvent (toluene) is 0.3°. Mole fraction elevation constant of non-polar solvent is 36.1 K. Thus, molality (mol kg-1) of the solution is
Which of the given aqueous solution has maximum boiling point?
(Kb of H20 = 0 .5 2 ° mol-1 kg), Tb (H20 ) = 373 K)
X and Y both are soluble in given solvent. Molar mass of the polymer Y is
Given, H20 ( / ) H20 ( g ) a t 373 K, ΔH° = 9.72 kcal mol-1 and the concentration of the sucrose in water is 0.1 M. Find the elevated boiling point of water.
Glucose solution is 20% by weight of solution. Its boiling point is ........
Given 1 molal urea solution boils at 100.52° C and water boils at 1 atm pressure,
Latent heat of fusion and latent heat of vaporisation of a solvent are in the ratio of 1: 6 and freezing point and boiling point (in Kelvin scale) are in the ratio of 2:3. Thus, ratio of elevation in boiling point to depression in freezing point of 1 molal solution is
One or More than One Options Correct Type
This section contains 2 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. Out of the following, colligative properties are
10% aqueous solution of a non-electrolyte solute of molar mass (m) has elevation in boiling point x°, then
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d)
Passage
Heavy water boils at 101.42°Cand its molal elevation constant ( Kb ) is 10% higher than that of pure water. Elevation in 0.1 molal aqueous urea solution is 0.052°.
Q. What is the molal elevation constant (in K mol-1 kg) of heavy water?
What is the latent heat of vaporisation of heavy water?
Matching List Type
Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct
Q. One molal aqueous sucrose solution boils at 100.52° C and water boils at 100° C. 5% aqueous solution (by mass of solvent) of non-electrolyte A B 2 boils at 100.26° C, 9% aqueous solution (by mass of solvent) of non-electrolyte A 2B boils at 100.52°C. Match the molar masses given in Column II with the species in Column l and select the answer from the codes given below.
Codes

One Integer Value Correct Type
This section contains 2 questions, when worked out will result in an integer value from 0 to 9 (both inclusive)
Q. A solution containing 3.975 g of sulphur in 100 g ofCS2 boils at 319.67 K. The boiling point of pure CS2 is 319.30 K.
Kb( CS2 ) = 2.406° mol-1 kg
If molecular state of sulphur is (S)x then what is the value of x?
An aqueous solution containing 28.4 g of non-volatile compound having the stoichiometric composition CnH2nOn in 100 g water boils at 101.24°Cat 1 atm pressure. Kf (H20 ) = 0.52° mol-1 kg and boiling point of H20 = 373 K. What is the value of n?
|
9 docs|1272 tests
|