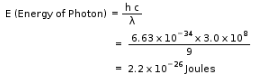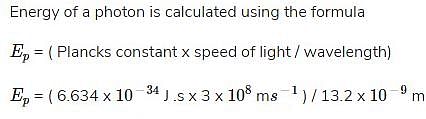Test: Planck’s Quantum Theory - JEE MCQ
15 Questions MCQ Test Chemistry for JEE Main & Advanced - Test: Planck’s Quantum Theory
The energy of a photon in Joules that has a wavelength of 9.0 m is:
The ratio of the energy of a photon of 2000 Ǻ wavelength radiation to that of 4000 Ǻ radiation is:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
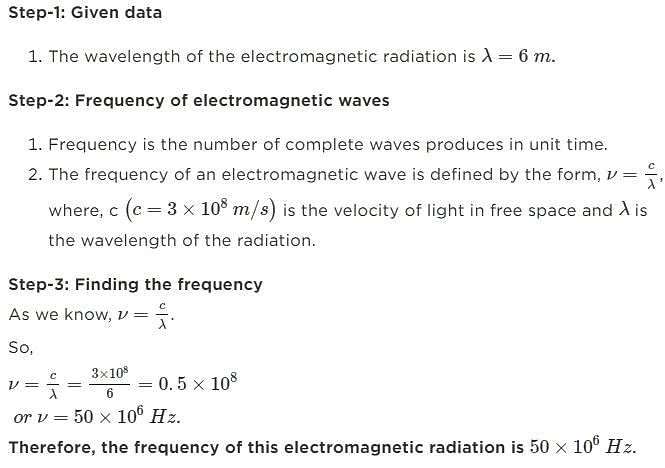
A radio operator broadcast on the 6-meter band. The frequency of this electromagnetic radiation is __________ MHz.
Electromagnetic radiation travels through vacuum at a speed of __________ m/s.
What is the frequency of light in s-1 that has a wavelength of 3.12 x 10-3 cm?
The ejected electrons from the surface of metal in photoelectric effect are called:
If the value of azimuthal quantum number is 3, the possible values of magnetic quantum numbers would be:
What is the energy of a photon that has a wavelength of 13.2 nm?
The wavelength of light that has a frequency of 1.20 × 1013s-1 is __________ m.
Which of the following radiation has the shortest wavelength.
Who discovered and first used the constant h = 6.6 x 10-34 J.s?
The energy of a photon of light has what kind of proportionality to its frequency and its wavelength.
What is the wavelength of light (nm) that has a frequency 4.62 ×1014s-1?
What is the frequency of a photon that has an energy of 3.7 x 10-18J?
|
352 videos|596 docs|309 tests
|
|
352 videos|596 docs|309 tests
|