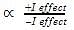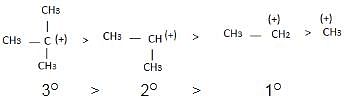NEET Exam > NEET Tests > Chemistry Class 11 > Test: Reaction Mechanism - NEET MCQ
Test: Reaction Mechanism - NEET MCQ
Test Description
10 Questions MCQ Test Chemistry Class 11 - Test: Reaction Mechanism
Test: Reaction Mechanism for NEET 2024 is part of Chemistry Class 11 preparation. The Test: Reaction Mechanism questions and answers have been
prepared according to the NEET exam syllabus.The Test: Reaction Mechanism MCQs are made for NEET 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Reaction Mechanism below.
Solutions of Test: Reaction Mechanism questions in English are available as part of our Chemistry Class 11 for NEET & Test: Reaction Mechanism solutions in
Hindi for Chemistry Class 11 course. Download more important topics, notes, lectures and mock
test series for NEET Exam by signing up for free. Attempt Test: Reaction Mechanism | 10 questions in 15 minutes | Mock test for NEET preparation | Free important questions MCQ to study Chemistry Class 11 for NEET Exam | Download free PDF with solutions
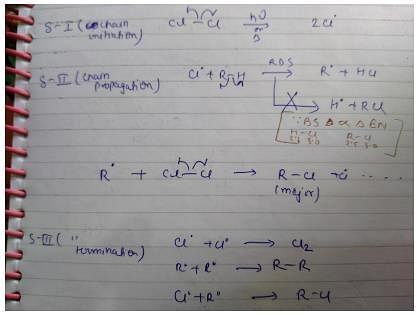
Detailed Solution for Test: Reaction Mechanism - Question 1
Test: Reaction Mechanism - Question 2
The observed order of the stability of the cabocation is:
Detailed Solution for Test: Reaction Mechanism - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Detailed Solution for Test: Reaction Mechanism - Question 3
Test: Reaction Mechanism - Question 4
A sequential account of each step, describing details of electron movement, energetics during bond cleavage and bond formation, and the rates of transformation of reactants into products (kinetics) is referred to as:
Detailed Solution for Test: Reaction Mechanism - Question 4
Detailed Solution for Test: Reaction Mechanism - Question 5
Test: Reaction Mechanism - Question 6
Which among the following is a very unstable and reactive species:
Detailed Solution for Test: Reaction Mechanism - Question 6
Detailed Solution for Test: Reaction Mechanism - Question 7
Test: Reaction Mechanism - Question 8
A carbon species carrying a negative charge on carbon atom is known as:
Detailed Solution for Test: Reaction Mechanism - Question 8
Test: Reaction Mechanism - Question 9
A species having a carbon atom possessing a sextet of electrons and a positive charge is called as:
Detailed Solution for Test: Reaction Mechanism - Question 9
Test: Reaction Mechanism - Question 10
The organic reaction which proceeds through heterolytic bond cleavage are known as:
Detailed Solution for Test: Reaction Mechanism - Question 10
|
129 videos|238 docs|88 tests
|
Information about Test: Reaction Mechanism Page
In this test you can find the Exam questions for Test: Reaction Mechanism solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Reaction Mechanism, EduRev gives you an ample number of Online tests for practice