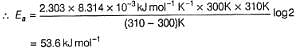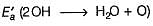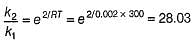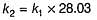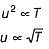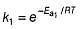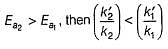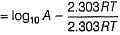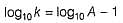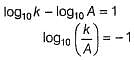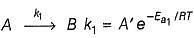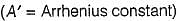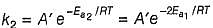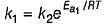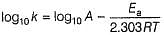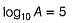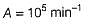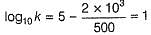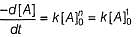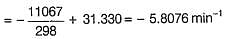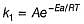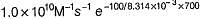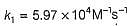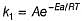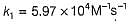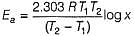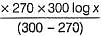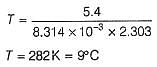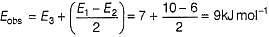Test: Energy of Activation - NEET MCQ
22 Questions MCQ Test Topic-wise MCQ Tests for NEET - Test: Energy of Activation
Direction (Q. Nos. 1-12) This section contains 12 multiple choice questions. Each question has four choices (a), (b), (c) and (cl), out of which ONLY ONE is correct.
Q.
The rate of a reaction doubles when its temperature changes from 300 K to 310 K. Activation energy of such a reaction will be (Given, R = 8.314 J K-1 mol-1, log2 = 0.3010).
In the following reaction
N2O 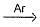 N2+O
N2+O
k = (5.0 x 1011 L mol-1 s-1) e -29000R/T, Ea (energy o f activation) is
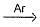 N2+O
N2+O| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Rate of a reaction can be expressed by Arrhenius equation as k = Ae-EalRT. In this equation, Ea represents.
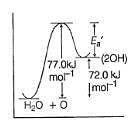
In the upper atmosphere, form ation of H2O2 takes place as
H2O+O → 2OH → H202
ΔH0 = 72.0 Kj mol-1 ; Ea = 77.0 kJ mol-1
Ea for the bim olecular recombination of two OH radicals to form H2O and O is
How much rate is affected in the presence of catalyst at 300 K?
For a reaction,
A → Product
t50 = 100 min at temprature T1
and t50 = 80 min at temprature T2
Thus,
Two reactions with different activation energies have the same rate at room temperature. Which statement correctly describes the rates of these two reactions at the same higher temperature?
The activation energy of the reaction at a given temperature is found to (2.303 RT) J mol-1. The ratio of rate constant to the Arrhenius factor is
From the given figure, select the correct statement about activation energy.
Activation energy of
The rate of a chemical reaction doubles for every 10°C rise of temperature. If the tem perature is raised by 50°C, the rate of the reaction increases by about
[AIEEE 2011]
A reactant A forms two products B and C as given
A 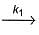 B, Activation energy Ea1
B, Activation energy Ea1
A 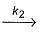 C, Activation energy Ea2
C, Activation energy Ea2
if Ea2 = 2Ea1, then k1 and K2 are related as
[AIEEE 2011]
Direction (Q. No. 14) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Rate constant {k) of a reaction is given by
Match the given property in Column I with its related value in Column II and select answer from codes given:
Direction (Q. Nos. 15-18)This section contains 2 paragraphs, which describing theory, experiments, data, etc. Four questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage I
For the displacement reaction,
[Co(NH3)5Cl]2+ (aq) + H2O (l) → [Co(NH3)5H2O]3+ + Cl- (aq)
The rate constant is given by
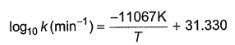
Q.
Rate constant at 25°C is
Passage I
For the displacement reaction,
[Co(NH3)5Cl]2+ (aq) + H2O (l) → [Co(NH3)5H2O]3+ + Cl- (aq)
The rate constant is given by
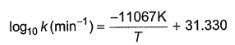
Q.
Energy of activation for this reaction is
Passage II
For the opposing reaction,
Ea (forward reaction) = 100 kJ mol-1
Pre-exponential factor A = 1.0 x 1010 M-1s-1

Q.
The value of k1 and k2 at 700K respectively are
Passage II
For the opposing reaction,
Ea (forward reaction) = 100 kJ mol-1
Pre-exponential factor A = 1.0 x 1010 M-1s-1

Q.
The rate constant (k1) for the forward reaction at 1000 K is
Direction (Q. Nos. 19-21) This section contains 3 questions. When worked out will result in one integer from 0 to 9 (both inclusive).
At room temperature (27°C) milk turns sour in about 64 h. In a refrigerator at -3°C, milk can be stored x times as long before it sours. The activation energy of the reaction that causes the souring of milk is 11.231 kcal mol-1.
What is the value of x?
The Arrhenius equations for the rate constant of decomposition of methyi nitrite and ethyl nitrite are
Find the temperature (in °C), at which rate contants are equal.
For the following sequence of reactions
Thus, observed Ea is .....kJ mol-1.
This section contains 2 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Select the correct statement(s) about collision theory of reaction rates.
Consider the endothermic reaction, X→Y
Heat o f reaction = ΔH with Ef = activation energy of the forw ard reaction.
Eb = activation energy of the backward reaction.
Then
|
9 docs|1272 tests
|