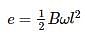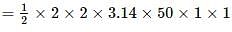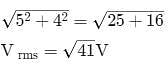Monthly Mock Test (February 29) - NEET MCQ
30 Questions MCQ Test Daily Test for NEET Preparation - Monthly Mock Test (February 29)
A voltage of peak value 283 V varying frequency is applied to a series L-C-R combination in which R = 3W; L = 25 mH and C = 400 mF. Then, the frequency (in Hz) of the source at which maximum power is dissipated in the above, is
What is the correct order of equilibrium enol content of the following compounds?
I. CH3COCH3
II. CH3COCH2COOC2H5
III. CH3COCH2COCH3
IV. CH3COCH2COH
II. CH3COCH2COOC2H5
III. CH3COCH2COCH3
IV. CH3COCH2COH
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The potential difference V and current i flowing through an a.c. circuit are given by V = 5 cos wt volt, i = 2 sin wt amp. the power dissipated in the circuit.
When 100 V DC is applied across a solenoid a current of 1A flows in it. When 100 V AC is applied across the same coil, the current drops to 0.5 A. If the frequency of the AC source is 50 Hz, the impedance and inductance of the solenoid are :
The two polypeptide chains in insulin are linked via Sulphur. The bond formed is
The cry1Ac gene in Bt Brinjal is under the transcriptional control of an enhanced cauliflower mosaic virus 35S (CaMV35S) promoter, which ensures
Seeds of golden rice are yellow in colour because
Which form s most stable acetal in the given condition?
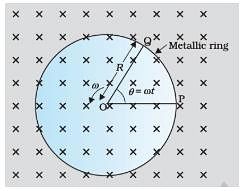
A metallic rod of 1 m length is rotated with a frequency of 50 rev/s about an axis passing through the centre point O. Other end of the metallic rod slides on a Metallic ring . A constant and uniform magnetic field of 2 T parallel to the axis is present everywhere. What is the emf between the centre and the metallic ring?
Cry genes introduced in a plant will not be affective against
What is the major product in the following reaction?
An ac generator consists of 8 turns of wire, each of area A=0.0900m2 , and the total resistance of the wire is 12.0Ω. The loop rotates in a 0.500-T magnetic field at a constant frequency of 60.0 Hz. Maximum induced emf is
Cry toxin is released from the crystal in the insect gut as
In the circuit shown if the emf of source at an instant is 5V, the potential difference across capacitor at the same instant is 4V. The potential difference across R at that instant may be
Assertion: Bacillus thuringiensis forms protein crystals during particular phase of growth.Reason: These crystals contain a toxic insecticidal protein that kills the certain insects.
Which of the following observation does not establish the existence of keto-enol tautomerism in acetone?
A person born with hereditary disease can be treated by
Coils C1 and C2 are stationary. C1 connected in series with one very small diwali bulb while C2 is connected to a battery .If the connection is suddenly snapped
Agrobacterium naturally inserts DNA into plants by
Among the following compounds, one that will not show keto-enol tautomerism is
The main challenge for production of insulin using rDNA technique was
Which of the following methods should be used to increase crop production without use of chemicals and fertilizers?
Biotechnology has advantages over conventional breeding as
What is the term used to refer to the use of bio-resources by multinational companies and other organizations without proper authorization from the countries and people concerned without contemporary payment is?
Arrange the following in the increasing order of acidic strength
I. H2CO
II. CH3CHO
III. C6H5CH2CHO
|
12 docs|366 tests
|