Test: Classification of Elements and Periodicity in Properties (June 9) - NEET MCQ
15 Questions MCQ Test Daily Test for NEET Preparation - Test: Classification of Elements and Periodicity in Properties (June 9)
Na+, Mg2+, Al3+ and Si4+ are isoelectronic. The order of their ionic size is [1993]
Which of the following sets has strongest tendency to form anions ? [1993]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The first ionization potentials (eV) of Be and B respectively are [1998]
The electronic configuration of four elements are given below. Which elements does not belong to the same family as others ? [1989]
Which of the following statements is true? [2002]
Identify the correct order of the size of the following: [2007]
Correct order of first IP among following elements Be, B, C, N, O is [2001]
Which one of the following ions will be the smallest in size? [1996]
If the atomic number of an element is 33, it will be placed in the periodic table in the
Which electronic configuration of an element has abnormally high difference between second and third ionization energy ? [1993]
The element, with atomic number 118, will be
Which of the following order is wrong? [2002]
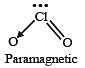
Which one of the following oxides is expected to exhibit paramagnetic behaviour? [2005]
One of the characteristic properties of non-metals is that they
In the periodic table, with the increase in atomic number, the metallic character of an element [1989]
|
12 docs|366 tests
|