Test: Kinetic Theory (October 14) - NEET MCQ
10 Questions MCQ Test Daily Test for NEET Preparation - Test: Kinetic Theory (October 14)
The number of degrees of freedom a diatomic molecule is
A 14.5 kg mass, fastened to the end of a steel wire of unstretched length 1.0 m, is whirled in a vertical circle with an angular velocity of 2 rev/s at the bottom of the circle. The cross-sectional area of the wire is 0.065 cm2. Calculate the elongation of the wire when the mass is at the lowest point of its path.
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The number of degrees of freedom a monatomic molecule is
One mole of an ideal monatomic gas is at an initial temperature of 300 K. The gas undergoes an isovolumetric process, acquiring 500 J of energy by heat. It then undergoes an isobaric process, losing this same amount of energy by heat. Determine the work done on the gas.
1 mole of a monoatomic gas is mixed with 3 moles of a diatomic gas. What is the molecular specific heat of the mixture at constant volume?
In which case are the atoms relatively rigidly fixed?
The average distance a molecule can travel without colliding is called the
In dynamic equilibrium, molecules collide and change their speeds during the collision
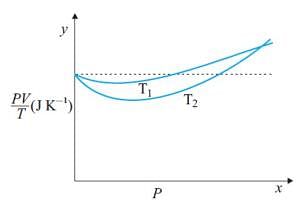
Figure shows plot of PV/T versus P for 1.00x10-3 kg of oxygen gas at two different temperatures. What is the value of PV/T where the curves meet on the y-axis?
|
12 docs|366 tests
|


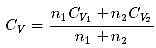
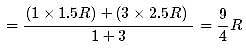
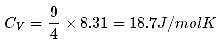
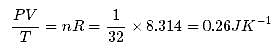 Hence the value of PV/T where the curves meet on the y-axis is 0.26 jK-1
Hence the value of PV/T where the curves meet on the y-axis is 0.26 jK-1














