NEET Exam > NEET Tests > Daily Test for NEET Preparation > Test: Nature of Matter & Its Properties (April 17) - NEET MCQ
Test: Nature of Matter & Its Properties (April 17) - NEET MCQ
Test Description
10 Questions MCQ Test Daily Test for NEET Preparation - Test: Nature of Matter & Its Properties (April 17)
Test: Nature of Matter & Its Properties (April 17) for NEET 2024 is part of Daily Test for NEET Preparation preparation. The Test: Nature of Matter & Its Properties (April 17) questions and answers have been
prepared according to the NEET exam syllabus.The Test: Nature of Matter & Its Properties (April 17) MCQs are made for NEET 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Nature of Matter & Its Properties (April 17) below.
Solutions of Test: Nature of Matter & Its Properties (April 17) questions in English are available as part of our Daily Test for NEET Preparation for NEET & Test: Nature of Matter & Its Properties (April 17) solutions in
Hindi for Daily Test for NEET Preparation course. Download more important topics, notes, lectures and mock
test series for NEET Exam by signing up for free. Attempt Test: Nature of Matter & Its Properties (April 17) | 10 questions in 20 minutes | Mock test for NEET preparation | Free important questions MCQ to study Daily Test for NEET Preparation for NEET Exam | Download free PDF with solutions
Test: Nature of Matter & Its Properties (April 17) - Question 1
Few quantities with their units are listed below. Mark the units which are not correctly matched.
(i) Density: kg m-3
(ii) Velocity of light: m s-1
(iii) Planck's constant: J-1 s-1
(iv) Acceleration: m s-2
(v) Force: kg m
(i) Density: kg m-3
(ii) Velocity of light: m s-1
(iii) Planck's constant: J-1 s-1
(iv) Acceleration: m s-2
(v) Force: kg m
Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 1
Test: Nature of Matter & Its Properties (April 17) - Question 2
Match the prefixes present in column I with their multiples in column II and mark the appropriate choice.
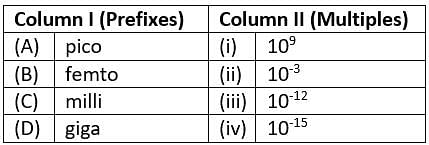

Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Nature of Matter & Its Properties (April 17) - Question 3
Choose the correct statement about I, II and III.
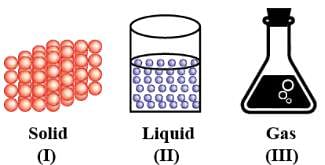

Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 3
Test: Nature of Matter & Its Properties (April 17) - Question 4
Mark the conversion factor which is not correct.
Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 4
Test: Nature of Matter & Its Properties (April 17) - Question 5
Consider the following figure,
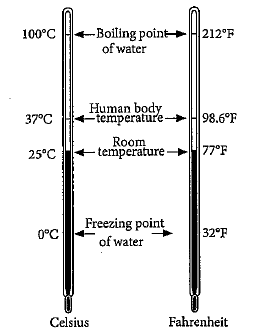
The correct relationship between fahrenheit and celsius scale is:
Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 5
Test: Nature of Matter & Its Properties (April 17) - Question 6
Which of the following is an element?
Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 6
Test: Nature of Matter & Its Properties (April 17) - Question 7
Which of the following is a compound?
Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 7
Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 8
Test: Nature of Matter & Its Properties (April 17) - Question 9
The correct relationship between picometer and nanometer is
Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 9
Test: Nature of Matter & Its Properties (April 17) - Question 10
Which one of the following is not a mixture?
Detailed Solution for Test: Nature of Matter & Its Properties (April 17) - Question 10
|
12 docs|366 tests
|
Information about Test: Nature of Matter & Its Properties (April 17) Page
In this test you can find the Exam questions for Test: Nature of Matter & Its Properties (April 17) solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Nature of Matter & Its Properties (April 17), EduRev gives you an ample number of Online tests for practice

















