Test: Nernst Equation Conductance of Electrolytic Solutions (November 11) - NEET MCQ
10 Questions MCQ Test Daily Test for NEET Preparation - Test: Nernst Equation Conductance of Electrolytic Solutions (November 11)
Cell reaction is spontaneous when
Gibbs free energy change for a cell reaction is positive what does it indicates?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In the equation, ΔG° = – nF E° cell ; F is:
Consider the cell reaction:
Cd(s) | Cd2+ (1.0 M) || Cu2+ (1.0 m) | Cu (s)
If we wish to make a cell with more positive voltage using the same substances, we should:
Conductivity (K) of 0.01 M NaCI solution is 0.00145 Scm-1. What happens to the conductivity if extra 100 mL of H2O be added to the above solution?
Specific conductance of 0.01 N KCI solution is x Scm-1 having conductance y S. Thus, specific conductance of 0.01 N NaCI having conductance zS is (in S cm-1)
500 mL of an aqueous solution contains 0.1 mole of KCl. If its specific conductance is x Scm-1, its molar conductance will be (in Scm2 mol-1)
Which quantity is temperature independent?
Given, (Scm2 mol-1)for different electrolytes
Thus, of CH3COOH is
At 298 K, given specific conductance of saturated
AgCl solution=3.41 x 10-6 Ω-1 cm-1 and that of water used =1.60 x 10-6 Ω-1 cm-1.
Equivalent conductance of saturated AgCl solution= 138.3 Ω-1 cm2 equiv-1
Thus, solubility product (KSp) of AgCl is
|
12 docs|366 tests
|


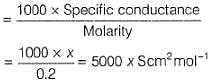
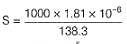

 Ag+ + Cl-
Ag+ + Cl-














