Test: Nomenclature of Coordination Compounds (December 25) - NEET MCQ
10 Questions MCQ Test Daily Test for NEET Preparation - Test: Nomenclature of Coordination Compounds (December 25)
The IUPAC name of the compound [Cr(NH3)5(NCS)][ZnCI4] is
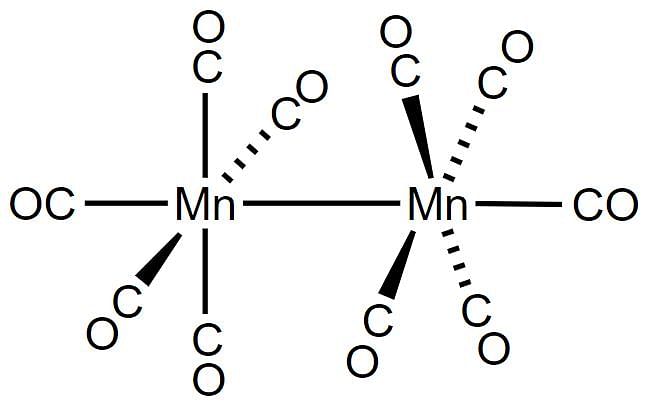
The IUPAC name of the complex [(CO)5Mn - Mn(CO)5] is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
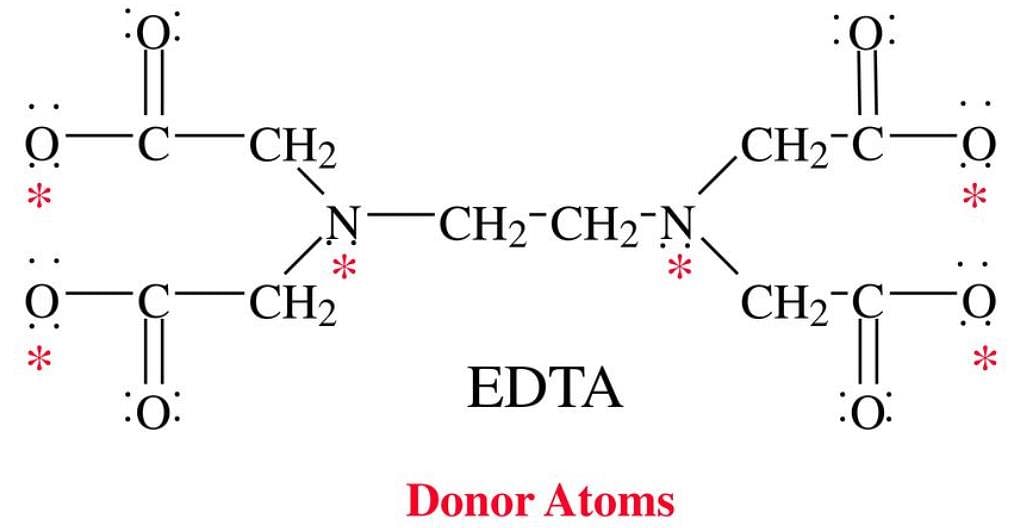
The donor sites of EDTA ligand are
What is the IUPAC name of compound NaBH4?
Direction (Q. Nos. 11-15) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Which of the following complexes have correct name?
Comprehension Type
Direction (Q. Nos. 16 and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions have been related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
In naming complexes, cation is named first followed by anion. Non-ionic or neutral complexes are given a one word name. The cationic and neutral complex ions are named by writing ‘the number and name of the ligands followed by the name of the central metal ion with its oxidation number in roman numerals within the parenthesis. If the complex is anionic, name of the metals ends with ‘ate’. When the complex contain two or more metal atoms linked with the help of bridging ligand then each bridging ligand is started with μ.
Q.
Name of [CoBr(NH3)4 NO2] is
In naming complexes, cation is named first followed by anion. Non-ionic or neutral complexes are given a one word name. The cationic and neutral complex ions are named by writing ‘the number and name of the ligands followed by the name of the central metal ion with its oxidation number in roman numerals within the parenthesis. If the complex is anionic, name of the metals ends with ‘ate’. When the complex contain two or more metal atoms linked with the help of bridging ligand then each bridging ligand is started with μ.
Q.
The IUPAC name of [Ni(NH3)4] [NiCI4] is
The oxidation state of the central metai in the complex ion [Fe(EDTA)]- .
The complex potassium dicyanodioxalatonickelate (II) in solution produce....... ions.
Formula of brown ring complex is
|
12 docs|366 tests
|