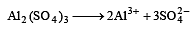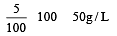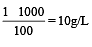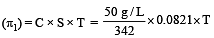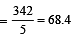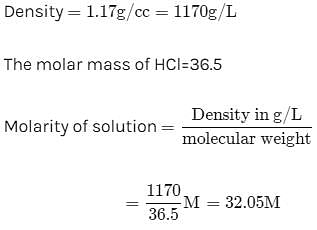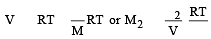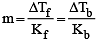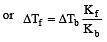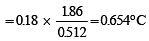Test: Solutions (October 22) - NEET MCQ
15 Questions MCQ Test Daily Test for NEET Preparation - Test: Solutions (October 22)
An ideal solution is formed when its components [1988]
The relative lowering of the vapour pressure is equal to the ratio between the number of [1991]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following modes of expressing concentration is independent of temperature ? [1992,1995]
If 0.1 M solution of glucose and 0.1 M solution of urea are placed on two sides of the semipermeable membrane to equal heights, then it will be correct to say that [1992]
Which one of the following salts will have the same value of van’t Hoff factor (i) as that of K4[Fe (CN)6]. [1994]
The number of moles of oxygen in one litre of air containing 21% oxygen by volume, in standard conditions, is [1995]
According to Raoult's law, relative lowering of vapour pressure for a solution is equal to [1995
Which of the following 0.10 m aqueous solutions will have the lowest freezing point ? [1997]
A 5% solution of cane sugar (mol. wt. =342) is isotonic with 1% solution of a substance X. The molecular weight of X is [1998]
Which of the following statements, regarding the mole fraction (x) of a component in solution, is incorrect? [1999]
The beans are cooked earlier in pressure cooker, because [2001]
Molarity of liquid HCl will be, if density of solution is 1.17 gm/cc [2001]
A solution con tains non -volatile solute of molecular mass M2. Which of the following can be used to calculate the molecular mass of solute in terms of osmotic pressure ? [2002]
Camphoris often used in molecular mass determination because [2004]
A solution of urea (m ol. mass 56 g mol-1) boils at 100.18°C at the atmospheric pressure. If Kf and Kb for water are 1.86 and 0.512 K kg mol-1 respectively, the above solution will freeze at [2005]
|
12 docs|366 tests
|


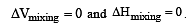
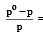 mole fraction of solute
mole fraction of solute 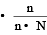

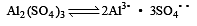
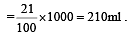
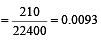
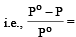 mole fraction of solute
mole fraction of solute