Test: Chemistry Minor Mock Test- 1 (March 16) - NEET MCQ
30 Questions MCQ Test Daily Test for NEET Preparation - Test: Chemistry Minor Mock Test- 1 (March 16)
Which electronic level would allow the hydrogen atom to absorb a photon but not to emit a photon:
Direction (Q. Nos. 1-15) This section contains 15 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Which of the following is least likely to behave as Lewis base?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following molecules are expected to exhibit intermolecular H-bonding
I. Acetic acid
II. o-nitrophenol
III. m-nitrophenol
IV. o-boric acid
Consider the following properties.


Select intensive and extensive properties.
The Value of (n2 + n1) and for He+ ion in atomic spectrum are 4 and 8 respectively. The wavelength of emitted photon when electron jump from n2 to n1 is
The critical temperature of water is higher than that of O2 because the H2O molecules has :
Among the alkali metals cesium is the most reactive because
For the reaction
Fe2 O3 (s) + 3 CO (g) → 2 Fe (g) + 3 CO2,
224 g of CO is available to react with 400 g Fe2O3, the yield of iron and CO2, are:
An element belongs to Group 15 and third period of the periodic table. Its electronic configuration will be
The third line in Balmer series corresponds to an electronic transition between which Bohr's orbits in hydrogen :
For an endothermic reaction when ΔH represents the enthalpy of the reaction in kJ mol-1, the minimum value for the energy of activation will be
[IIT JEE 1992]
56 g of iron reacts with dilute H2SO4 at 27° C . W ork done (in cals) in
I. closed vessel of fixed volume and
II. an open vessel is


A measured temperature is 1000F on Fahrenheit scale, then what is this reading be on Celsius scale:
For the following process, H2 (g) → 2 H(g), it absorbs 436 kJ mol-1. Thus,
The light radiations with discrete quantities of energy are called ___________ .
Compound with maximum ionic character is formed from :
Suppose the elements X and Y combine to form two compounds XY2 and X3Y2. When 0.1 mole of XY2 weighs 10 g and 0.05 mole of X3Y2 weighs 9 g, the atomic weights of X and Y are
In the following reaction,
Species behaving as Bronsted-Lowry acids are
The energy of electron is maximum at :
Which of the following will produce a buffer solution when mixed in equal volumes ?
Direction (Q. Nos. 1-15) This section contains 15 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q.
Assign the sign of work done (based on SI convention) in the following chemical changes taking place against external atmospheric pressure :
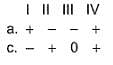
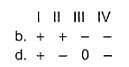
Choose one of the following in th increasing order of bond length
Which one of the following molecular hydrides acts as a Lewis acid?
The orbital angular momentum of an electron in 2s orbital is :
One mole of an ideal gas is put through a series of changes as shown in the figure in which 1,2,3 mark the three stages of the system. Pressure at the stages 1, 2, and 3 respectively will be (in bar)


The ratio of the energy of a photon of 2000 Å wavelength radiation to that of 4000 Å radiation is
SI units for Base Physical Quantities of length, mass and current are
|
12 docs|366 tests
|


















