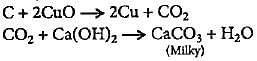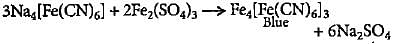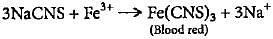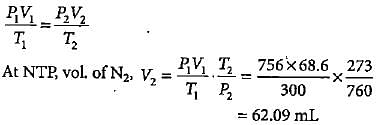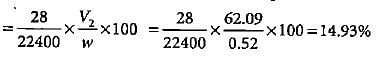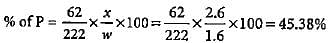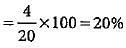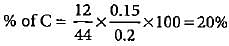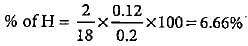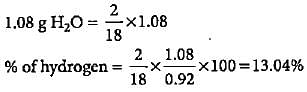Test: Qualitative & Quantitative Analysis of Organic Compounds (September 19) - NEET MCQ
10 Questions MCQ Test Daily Test for NEET Preparation - Test: Qualitative & Quantitative Analysis of Organic Compounds (September 19)
The presence of carbon in an organic compound can be shown by
The blue compound formed in the positive test for nitrogen with Lassaigne solution of an organic compound is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
If on adding FeCl3 solution to acidified Lassaigne solution, a blood red colouration is produced, it indicates the presence of
During sodium extract preparation for Lassaigne's test both N and S present in organic compound change to
In Duma'smethod 0.52 g of an organic compound on combustion gave 68.6 mL N2 at 27oC and 76 mm pressure. What is the percentage of nitrogen in the compound?
In kjeldahl's method of estimation of nitrogen, nitrogen is quantitatively converted to ammonium sulphate. It is the treated with the standard solution of alkali. The nitrogen which is present is estimated as:
1.6 g of an organic compound gave 2.6 g of magnesium pyrophosphate. The percentage of phosphorus in the compound is
The percentage of oxygen in heavy water is
0.2 g of an organic compound contains C, H and O.On combustion, it yields 0.15 g CO2 and 0.12 g H2O.Ihe percentage of C, H and O respectively is
0.92 g of an organic compound was analysed by combustion method. The mass of the U-tube increased by 1.08 g. What is the percentage of hydrogen in the compound?
|
12 docs|366 tests
|