Test: Chemistry Minor Mock Test- 3 (March 22) - NEET MCQ
30 Questions MCQ Test Daily Test for NEET Preparation - Test: Chemistry Minor Mock Test- 3 (March 22)
Directions: In this question, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion (A): The two strands of DNA are complementary to each other.
Reason (R): The hydrogen bonds are formed between specific pairs of bases.
Assertion (A): The two strands of DNA are complementary to each other.
Reason (R): The hydrogen bonds are formed between specific pairs of bases.
Which of the following ion has smallest radii?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Primary alcohols are prepared by reduction of carboxylic acids. Though lithium aluminium hydride is a strong reducing agent, it is not used in the reaction. Because
Which among the following is not considered as a part of transition elements?
 is a tertiary amine having IUPAC name as:
is a tertiary amine having IUPAC name as:
Propanamide on treatment with bromine in an aqueous solution of sodium hydroxide gives:
Directions: This question consist of two statements, each printed as Assertion and Reason. While answering this question, you are required to choose any one of the following four responses.
Assertion : Vitamin D cannot be stored in our body
Reason : Vitamin D is fat soluble vitamin and is excreted from the body in urine
Which is the most stable oxidation state of iron?
Directions: In this question, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion (A): Glycine must be taken through diet.
Reason (R): It is non-essential amino acid.
Ketones are reduced to the corresponding alcohols by catalytic hydrogenation to form
What is the correct order of reactivity of alcohols in the following reaction?
A compond A has the molecular formula C5H9CI. It does not react with bromine in crabon tetrachloride. On treatement with strong base it produces a single compound B. B shas a molecular formula C5H8 and reacts with bromine in carbon tetrachloride. ozonolysis of B produces a compound cC which has a molecular formula C5H8O2. Which of the following structures is that of A?
Ketones react with Grignard reagent to produce
Nitro compounds are reduced to amines. The catalyst that is preferred is:
In which of the following complexes, the nickel metal is in the highest oxidation state?
Which reaction can be used for the direct conversion of amides into 10 amine ?
Which of the following reagents can be used to oxidise primary alcohols to aldehydes?
Maximum oxidation state is shown by
Which among the following transition metal has lowest melting point?
The oxidation state of Ag in tollen’s reagent is
The IUPAC name [CoCl(NO2)(en)2]Cl is
Directions: In this question, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
Assertion (A): Deoxyribose, C5H10O4 is not a carbohydrate.
Reason (R): Carbohydrates are optically active polyhydroxy aldehyde or polyhydroxy ketone or substances which give aldehyde or ketone on hydrolysis.
Which one of the following compound is deliquescent?
Transition metals with highest melting point is
Which of the following complex will give white precipitate with barium chloride solution?
|
12 docs|366 tests
|


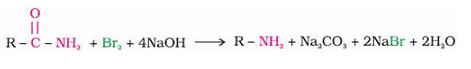
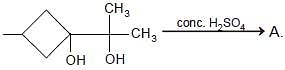 The product is -
The product is -