Test: Methods of Preparation of Haloalkanes and Haloarenes (January 25) - NEET MCQ
10 Questions MCQ Test Daily Test for NEET Preparation - Test: Methods of Preparation of Haloalkanes and Haloarenes (January 25)
On free radical chlorination reaction of butane, how many different, optically active, dichloroalkanes would be formed ?
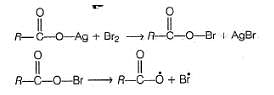
The yield of alkyl bromide obtained as a result of heating the dry silver salt of carboxylic acid with bromine in CCI4 is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which is incorrect about Hunsdiecker's reaction?
One or More than One Options Correct Type
Direction (Q. Nos. 8-12) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. Which of the following reagents can bring about free radical chlorination of propane?
Choose the correct statement(s) from the following regarding free radical chlorination and bromination reaction of alkane.
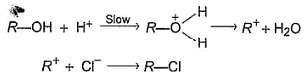
An alcohol (R — OH) can be converted into alkyl chloride by the treatm ent with HCI. Reaction involves protonation of alcohol followed by the formation of carbocation intermediate. Carbocation intermediate in the final step undergo nucleophilic attack by Cl- ion as :
Q.
What is the correct order of reactivty of the followings with HCl?
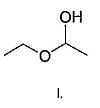
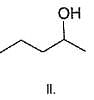
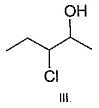
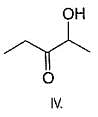
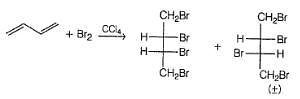
If 1, 3-butadiene is treated with excess of bromine in CCI4 , how many different tetrabromides would be formed?
Consider the following reaction,
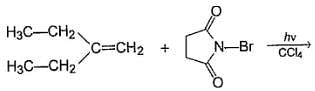
Q.
How many different monobromo derivatives would be produced?
Addition of bromine on propene in the presence of brine yields a mixture of
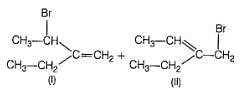
The major product of the following reaction is
|
12 docs|366 tests
|