Benzene: Resonance, Aromaticity, Preparation & Properties - NEET MCQ
22 Questions MCQ Test Topic-wise MCQ Tests for NEET - Benzene: Resonance, Aromaticity, Preparation & Properties
Direction (Q. Nos. 1 - 8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Q. Predict major product in the following reaction.
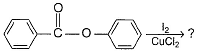
What is the major organic product in the following reaction?

| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
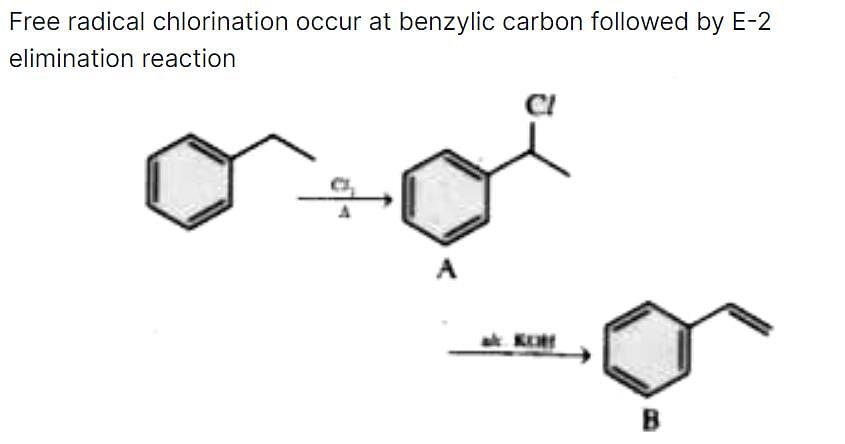
Ethyl benzene on heating with Cl2 gives A(C8H9CI). A on reaction with alcoholic KOH gives B (C8H8). Compounds A and 6 are, respectively
Benzene is aromatic because it follows
What is the major product of the following reaction?
Predict major product of the reaction below.
Provide the appropriate sequence of reagents that can bring about the following transformation.
Direction (Q. Nos. 9 - 14) This section contains 6 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q. Which of the following com pound(s) fail in Friedel-Crafts alkylation reaction?
What is/are true about the following reaction?
Consider the following reaction.
Q. What are the expected product(s)?
Consider the following reaction.
The correct statement(s) concerning X and Y is/are
What is/are true regarding Friedel-Crafts methylation reaction of phenol using CH3CI/AICI3?
What is /are the principal products of the following reaction ?
Direction (Q. Nos. 15 - 20) This section contains 2 paragraphs, each describing theory, experiments, data, etc. Six questions related to the paragraphs have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage I
An aromatic hydrocarbon P has molecular formula C9H12. P on treatment with alkaline KMnO4 in boiling condition followed by hydrolysis gives polar C8H6O4 . Also, P on treatment with CI2/AICI3 gives only two isomers Q and R as major products with their molecular formula C9H11CI.
Q. The most likely structure of P is
Passage I
An aromatic hydrocarbon P has molecular formula C9H12. P on treatment with alkaline KMnO4 in boiling condition followed by hydrolysis gives polar C8H6O4 . Also, P on treatment with CI2/AICI3 gives only two isomers Q and R as major products with their molecular formula C9H11CI
Q. Which of the following reaction represent best preparation of P?
Passage I
An aromatic hydrocarbon P has molecular formula C9H12. P on treatment with alkaline KMnO4 in boiling condition followed by hydrolysis gives polar C8H6O4 . Also, P on treatment with CI2/AICI3 gives only two isomers Q and R as major products with their molecular formula C9H11CI
Q. If all the monochloro isomers of P are considered, how many of them are possible?
Passage II
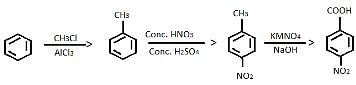
Consider the following road-map reaction,
Q. The most likely structure of P is
Passage II
Consider the following road-map reaction,
Q. What is Q?
Passage II
Consider the following road-map reaction,
Q. What is R ?
Direction (Q. Nos. 21 and 22) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Q. Match the reactions from Column I with expected products from Column II.
Match the reactions from Column I with expected product(s) from Column II.
|
9 docs|1272 tests
|