Case Based Questions Test: Aldehydes, Ketones & Carboxylic Acids - NEET MCQ
12 Questions MCQ Test Topic-wise MCQ Tests for NEET - Case Based Questions Test: Aldehydes, Ketones & Carboxylic Acids
Read the passage given below and answer the following questions:
Reduction of carboxylic acids and their derivatives plays an important role in organic synthesis, in both laboratory and industrial processes. Traditionally, the reduction is performed using stoichiometric amounts of hydride reagents, generating stoichiometric amounts of waste. A much more attractive, atom-economical approach is a catalytic reaction using H2; however, hydrogenation of carboxylic acid derivatives under mild conditions is a very challenging task, with amides presenting the highest challenge among all classes of carbonyl compounds. Very few examples of the important hydrogenation of amides to amines, in which the C-O bond is cleaved with the liberation of water (Scheme 1), were reported. C-O cleavage of amides can also be affected with silanes as reducing agents. (Generation of amides to the with cleavage of the C–N products of C–O cleavage the case of anilides). The and neutral, homogeneous Scheme 1. General Sche C-O cleavage We have now prepared the new, dearomatized, bipyridine-based pincer complex 3, catalyst 3 (Here refered as Cat. 3). Remarkably, it efficiently catalyzes the selective hydrogenation of amides to form amines and alcohols (eq 1). The reaction proceeds under mild pressure and neutral conditions, with no additives being required. Since the reaction proceeds well under anhydrous conditions, hydrolytic cleavage of the amide is not involved in this process. been reported.6 Amines and chemical, pharmaceutical and ch a reaction is conceptually step in amide hydrogenation bonvl group to form a very anhydrous condition involved in this pro

In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage.
Assertion (A): The use of catalyst 3 is an efficient method of preparation of primary amines
Reason (R): Use of catalyst 3 is a step down reaction.

Read the passage given below and answer the following questions:
Reduction of carboxylic acids and their derivatives plays an important role in organic synthesis, in both laboratory and industrial processes. Traditionally, the reduction is performed using stoichiometric amounts of hydride reagents, generating stoichiometric amounts of waste. A much more attractive, atom-economical approach is a catalytic reaction using H2; however, hydrogenation of carboxylic acid derivatives under mild conditions is a very challenging task, with amides presenting the highest challenge among all classes of carbonyl compounds. Very few examples of the important hydrogenation of amides to amines, in which the C-O bond is cleaved with the liberation of water (Scheme 1), were reported. C-O cleavage of amides can also be affected with silanes as reducing agents. (Generation of amides to the with cleavage of the C–N products of C–O cleavage the case of anilides). The and neutral, homogeneous Scheme 1. General Sche C-O cleavage We have now prepared the new, dearomatized, bipyridine-based pincer complex 3, catalyst 3 (Here refered as Cat. 3). Remarkably, it efficiently catalyzes the selective hydrogenation of amides to form amines and alcohols (eq 1). The reaction proceeds under mild pressure and neutral conditions, with no additives being required. Since the reaction proceeds well under anhydrous conditions, hydrolytic cleavage of the amide is not involved in this process. been reported.6 Amines and chemical, pharmaceutical and ch a reaction is conceptually step in amide hydrogenation bonvl group to form a very anhydrous condition involved in this pro
In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage.
Assertion (A): N-methyl ethanamide on reaction with catalyst 3 will yield ethanol and methanamine.
Reason (R): Use of Catalyst 3 brings about cleavage of C-N bond of amides
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Read the passage given below and answer the following questions:
Reduction of carboxylic acids and their derivatives plays an important role in organic synthesis, in both laboratory and industrial processes. Traditionally, the reduction is performed using stoichiometric amounts of hydride reagents, generating stoichiometric amounts of waste. A much more attractive, atom-economical approach is a catalytic reaction using H2; however, hydrogenation of carboxylic acid derivatives under mild conditions is a very challenging task, with amides presenting the highest challenge among all classes of carbonyl compounds. Very few examples of the important hydrogenation of amides to amines, in which the C-O bond is cleaved with the liberation of water (Scheme 1), were reported. C-O cleavage of amides can also be affected with silanes as reducing agents. (Generation of amides to the with cleavage of the C–N products of C–O cleavage the case of anilides). The and neutral, homogeneous Scheme 1. General Sche C-O cleavage We have now prepared the new, dearomatized, bipyridine-based pincer complex 3, catalyst 3 (Here refered as Cat. 3). Remarkably, it efficiently catalyzes the selective hydrogenation of amides to form amines and alcohols (eq 1). The reaction proceeds under mild pressure and neutral conditions, with no additives being required. Since the reaction proceeds well under anhydrous conditions, hydrolytic cleavage of the amide is not involved in this process. been reported.6 Amines and chemical, pharmaceutical and ch a reaction is conceptually step in amide hydrogenation bonvl group to form a very anhydrous condition involved in this pro
In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage.
Assertion (A): Use of hydride catalyst or hydrogen brings about cleavage of C-O bond in amides.
Reason (R): Hydride catalyst or hydrogen cause to reduction of amides.
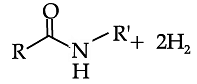
Read the passage given below and answer the following questions:
Reduction of carboxylic acids and their derivatives plays an important role in organic synthesis, in both laboratory and industrial processes. Traditionally, the reduction is performed using stoichiometric amounts of hydride reagents, generating stoichiometric amounts of waste. A much more attractive, atom-economical approach is a catalytic reaction using H2; however, hydrogenation of carboxylic acid derivatives under mild conditions is a very challenging task, with amides presenting the highest challenge among all classes of carbonyl compounds. Very few examples of the important hydrogenation of amides to amines, in which the C-O bond is cleaved with the liberation of water (Scheme 1), were reported. C-O cleavage of amides can also be affected with silanes as reducing agents. (Generation of amides to the with cleavage of the C–N products of C–O cleavage the case of anilides). The and neutral, homogeneous Scheme 1. General Sche C-O cleavage We have now prepared the new, dearomatized, bipyridine-based pincer complex 3, catalyst 3 (Here refered as Cat. 3). Remarkably, it efficiently catalyzes the selective hydrogenation of amides to form amines and alcohols (eq 1). The reaction proceeds under mild pressure and neutral conditions, with no additives being required. Since the reaction proceeds well under anhydrous conditions, hydrolytic cleavage of the amide is not involved in this process. been reported.6 Amines and chemical, pharmaceutical and ch a reaction is conceptually step in amide hydrogenation bonvl group to form a very anhydrous condition involved in this pro
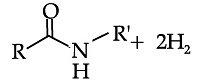
In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage.
Assertion (A): Aniline can be prepared from suitable amide using catalyst 3
Reason (R): The use of catalyst 3 is limited to aliphatic amides only.
Read the passage given below and answer the following questions:
Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl groups. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by treatment of Grignard reagents with carbon dioxide.
Q. Name a method by which both aldehydes and ketones can be prepared.
Read the passage given below and answer the following questions:
Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl groups. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by treatment of Grignard reagents with carbon dioxide.
Q. The reagent which does not react with both, acetone and benzaldehyde.
Read the passage given below and answer the following questions:
Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl groups. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by treatment of Grignard reagents with carbon dioxide.
Q. How will you distinguish between aliphatic aldehydes and aromatic aldehydes?
Read the passage given below and answer the following questions:
Aldehydes, ketones and carboxylic acids are few of the major classes of organic compounds containing carbonyl groups. Aldehydes are prepared by dehydrogenation or controlled oxidation of primary alcohols and controlled or selective reduction of acyl halides. Ketones are prepared by oxidation of secondary alcohols and hydration of alkynes. Carboxylic acids are prepared by the oxidation of primary alcohols, aldehydes and alkenes by hydrolysis of nitriles and by treatment of Grignard reagents with carbon dioxide.
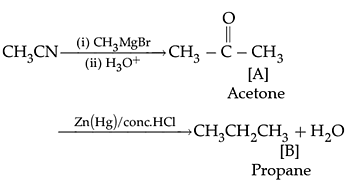
Q. Name the main compounds A and B formed in the following reaction:![]()
Read the passage given below and answer the following questions:
Reductive alkylation is the term applied to the process of introducing alkyl groups into ammonia or a primary or secondary amine by means of an aldehyde or ketone in the presence of a reducing agent. The present discussion is limited to those reductive alkylations in which the reducing agent is hydrogen and a catalyst or "nascent" hydrogen, usually from a metalacid combination; most of these reductive alkylations have been carried out with hydrogen and a catalyst. The principal variation excluded is that in which the reducing agent is formic acid or one of its derivatives; this modification is known as the Leuckart reaction. The process of reductive alkylation of ammonia consists in the addition of ammonia to a carbonyl compound and reduction of the addition compound or its dehydration product. The reaction usually is carried out in ethanol solution when the reduction is to be effected catalytically:

Since the primary amine is formed in the presence of the aldehyde it may react in the same way as ammonia, yielding an additional compound, a Schiff's base (RCH= NCH2R) and finally, a secondary amine. Similarly, the primary amine may react with the imine, forming an addition product which also is reduced to a secondary amine Finally, the secondary amine may react with either the aldehyde or the imine to give products which are reduced to tertiary amines.
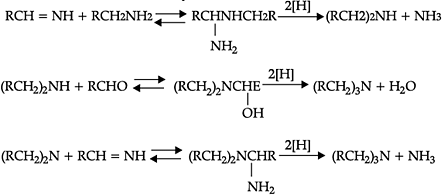
Similar reactions may occur when the carbonyl compound employed is a ketone.
Q. Ethanal on reaction with ammonia forms an imine (X) which on reaction with nascent hydrogen gives (Y). Identify ‘X’ and ‘Y’.
Read the passage given below and answer the following questions:
Reductive alkylation is the term applied to the process of introducing alkyl groups into ammonia or a primary or secondary amine by means of an aldehyde or ketone in the presence of a reducing agent. The present discussion is limited to those reductive alkylations in which the reducing agent is hydrogen and a catalyst or "nascent" hydrogen, usually from a metalacid combination; most of these reductive alkylations have been carried out with hydrogen and a catalyst. The principal variation excluded is that in which the reducing agent is formic acid or one of its derivatives; this modification is known as the Leuckart reaction. The process of reductive alkylation of ammonia consists in the addition of ammonia to a carbonyl compound and reduction of the addition compound or its dehydration product. The reaction usually is carried out in ethanol solution when the reduction is to be effected catalytically:

Since the primary amine is formed in the presence of the aldehyde it may react in the same way as ammonia, yielding an additional compound, a Schiff's base (RCH= NCH2R) and finally, a secondary amine. Similarly, the primary amine may react with the imine, forming an addition product which also is reduced to a secondary amine Finally, the secondary amine may react with either the aldehyde or the imine to give products which are reduced to tertiary amines.

Similar reactions may occur when the carbonyl compound employed is a ketone.
Q. ![]()
The compound Q is:
Read the passage given below and answer the following questions:
Reductive alkylation is the term applied to the process of introducing alkyl groups into ammonia or a primary or secondary amine by means of an aldehyde or ketone in the presence of a reducing agent. The present discussion is limited to those reductive alkylations in which the reducing agent is hydrogen and a catalyst or "nascent" hydrogen, usually from a metalacid combination; most of these reductive alkylations have been carried out with hydrogen and a catalyst. The principal variation excluded is that in which the reducing agent is formic acid or one of its derivatives; this modification is known as the Leuckart reaction. The process of reductive alkylation of ammonia consists in the addition of ammonia to a carbonyl compound and reduction of the addition compound or its dehydration product. The reaction usually is carried out in ethanol solution when the reduction is to be effected catalytically:

Since the primary amine is formed in the presence of the aldehyde it may react in the same way as ammonia, yielding an additional compound, a Schiff's base (RCH= NCH2R) and finally, a secondary amine. Similarly, the primary amine may react with the imine, forming an addition product which also is reduced to a secondary amine Finally, the secondary amine may react with either the aldehyde or the imine to give products which are reduced to tertiary amines.

Similar reactions may occur when the carbonyl compound employed is a ketone.
Q. Acetaldehyde is reacted with ammonia followed by reduction in presence of hydrogen as a catalyst. The primary amine so formed further reacts with acetaldehyde. The Schiff’s base formed during the reaction is:
Read the passage given below and answer the following questions:
Reductive alkylation is the term applied to the process of introducing alkyl groups into ammonia or a primary or secondary amine by means of an aldehyde or ketone in the presence of a reducing agent. The present discussion is limited to those reductive alkylations in which the reducing agent is hydrogen and a catalyst or "nascent" hydrogen, usually from a metalacid combination; most of these reductive alkylations have been carried out with hydrogen and a catalyst. The principal variation excluded is that in which the reducing agent is formic acid or one of its derivatives; this modification is known as the Leuckart reaction. The process of reductive alkylation of ammonia consists in the addition of ammonia to a carbonyl compound and reduction of the addition compound or its dehydration product. The reaction usually is carried out in ethanol solution when the reduction is to be effected catalytically:

Since the primary amine is formed in the presence of the aldehyde it may react in the same way as ammonia, yielding an additional compound, a Schiff's base (RCH= NCH2R) and finally, a secondary amine. Similarly, the primary amine may react with the imine, forming an addition product which also is reduced to a secondary amine Finally, the secondary amine may react with either the aldehyde or the imine to give products which are reduced to tertiary amines.
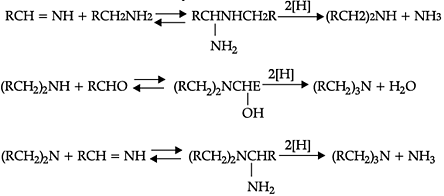
Similar reactions may occur when the carbonyl compound employed is a ketone.
Q. The reaction of ammonia and its derivatives with aldehydes is called:
|
9 docs|1272 tests
|