Test: Ambident Nucleophiles & NGP in SN2 Reactions - NEET MCQ
19 Questions MCQ Test Chemistry Class 12 - Test: Ambident Nucleophiles & NGP in SN2 Reactions
Only One Option Correct Type
Direction (Q. Nos. 1-8) This section contains 8 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
Cyanide anion has two atoms th at have lone pair of electrons. Either could act as nucleophile in the reaction. Yet in the vast majority of the cases, carbon acts as nucleophile and forms a bond to the substrate, why?
In the following two phase reaction, catalyst works by
C6H5CH2Br + KCN 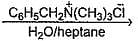 C6H5CH2CN + KBr
C6H5CH2CN + KBr
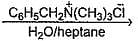 C6H5CH2CN + KBr
C6H5CH2CN + KBr| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following would react most rapidly with sodium ethoxide to produce an ether?
In the given reaction,
X will be
In the given reaction,
X will be
Consider the following reaction,
Q.
What can be correctly predicted regarding this reaction?
Pick out the alkyl bromide which proceeds with retention of configuration in an SN2 reaction with CH3ONa (aq).
For the following reaction, pick out the best term which describe its mechanism
One or More than One Options Correct Type
Direction (Q. Nos. 9-13) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Pick out the alkyl bromides which proceeds with retention of configuration in an SN2 reaction with CH3ONa(aq)
Which of the following is/are ambident nucleophile?
Which of the following gives the same substitution (SN2) product with C2H5Br no matter whether sodium or silver salt is used?
Which of the following gives the different substitution product with CH3CH2Br when their sodium and silver salts are used?
Consider the following substitution reaction,
Q.
The correct statement(s) is/are
Comprehension Type
Direction (Q. Nos. 14-16) This section contains a paragraph, describing theory, experiments, data, etc.
Three questions related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
Nucleophiles which have more than one type of donor atoms within the same donor group are known as ambident nucleophile e.g.
CN- : Both carbon and nitrogen are donor.
OCN- : Both oxygen and nitrogen are donor etc.
In case of free anionic and ambident nucleophiles, the better donor atom donate its lone pair of electron and forms bond with a-carbon after substitution reaction, in a given period, donor ability decreases from left to right. In a group, donor ability increases from top to bottom.
Q.
When CH3CH2Br is treated with aqueous NaCN, CH3CH2CN is formed while CH3CH2NCis formed when AgCN is used in the similar reaction. It is due to
Nucleophiles which have more than one type of donor atoms within the same donor group are known as ambident nucleophile e.g.
CN- : Both carbon and nitrogen are donor.
OCN- : Both oxygen and nitrogen are donor etc.
In case of free anionic and ambident nucleophiles, the better donor atom donate its lone pair of electron and forms bond with a-carbon after substitution reaction, in a given period, donor ability decreases from left to right. In a group, donor ability increases from top to bottom.
Q.
Treatment of CH3CH2Br with either NaNO2 or AgNO2 gives the same nitroethane as major product because
Nucleophiles which have more than one type of donor atoms within the same donor group are known as ambident nucleophile e.g.
CN- : Both carbon and nitrogen are donor.
OCN- : Both oxygen and nitrogen are donor etc.
In case of free anionic and ambident nucleophiles, the better donor atom donate its lone pair of electron and forms bond with a-carbon after substitution reaction, in a given period, donor ability decreases from left to right. In a group, donor ability increases from top to bottom.
Q.
When iodomethane is treated with sodium isocyanate (NaNCO), CH3NCO is the major product while AgNCO gives CH3CH2OCN as major product because
One Integer Value Correct Type
Direction (Q. Nos. 17 and 18) This section contains 2 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q.
If meso form of the 2,4-dibromopentane is treated with excess of KCN(aq), how many SN2 products with both bromide substituted, wouid result?
Consider the foiiowing SN2 reactions.
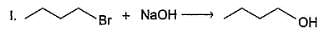
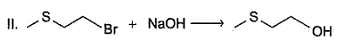
Q.
Only neighbouring effect increases reactivity of (II) by a factor of 5 x 104. If reaction (I) is carried out with initial concentration of C4H9Br = 0.05 M and NaOH 0.01 M and reaction (II) is carried out with initial concentration of both CH3SCH2CH2Br and NaOH = 0.1 M each, rate of reaction (II) would be 10x times greater than the rate of reaction (I) under identical experimental condition. What is the value of x?
Matching List Type
Direction (Q. No. 19) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Q.
Match the Column I with Column II and mark the correct option from the codes given below.
|
100 videos|282 docs|123 tests
|