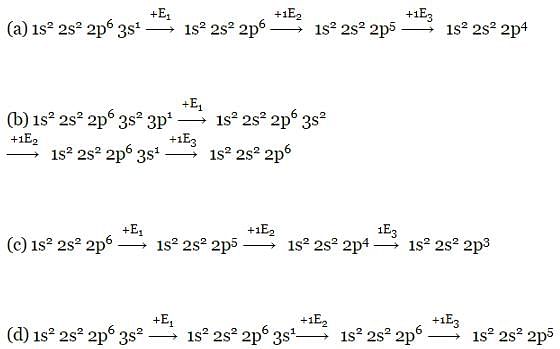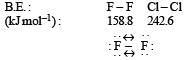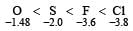31 Years NEET Previous Year Questions: Classification of Elements & Periodicity in Properties - NEET MCQ
30 Questions MCQ Test Chemistry Class 11 - 31 Years NEET Previous Year Questions: Classification of Elements & Periodicity in Properties
The electronic configuration of four elements are given below. Which elements does not belong to the same family as others ? [1989]
In the periodic table, with the increase in atomic number, the metallic character of an element [1989]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Elements of which of the following groups will form anions most readily ? [1992]
Which of the following sets has strongest tendency to form anions ? [1993]
One would expect proton to have very large
Na+, Mg2+, Al3+ and Si4+ are isoelectronic. The order of their ionic size is [1993]
If the atomic number of an element is 33, it will be placed in the periodic table in the
In the periodic table from left to right in a period, the atomic volume [1993]
Which electronic configuration of an element has abnormally high difference between second and third ionization energy ? [1993]
One of the characteristic properties of non-metals is that they
The electronic configuration of an element is 1s2 2s2 2p6 3s2 3p3 . What is the atomic number of the element, which is just below the above element in the periodic table? [1995]
The element, with atomic number 118, will be
Which one of the following ions will be the smallest in size? [1996]
Which of the following does not represent the correct order of the properties indicated [1997]
The first ionization potentials (eV) of Be and B respectively are [1998]
Of the given electronic configurations for the elements, which electronic configuration indicates that there will be abnormally high difference in the second and third ionization energy for the element? [1999]
Correct order of first IP among following elements Be, B, C, N, O is [2001]
Which of the following order is wrong? [2002]
An atom has electronic configuration 1s2 2s2 2p6 3s2 3p6 3d3 4s2, you will place it in which group?
Which of the following statements is true? [2002]
Among K, Ca, Fe and Zn the element which can form more than one binary compound with chlorine is [2004]
Ionic radii are [2004]
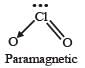
Which one of the following oxides is expected to exhibit paramagnetic behaviour? [2005]
Which one of the following arrangements represents the correct order of electron gain enthalpy (with negative sign) of the given atomi c species? [2 00 5]
Identify the correct order of the size of the following: [2007]
Which of the following electronic configuration an atom has the lowest ionisation enthalpy?
Which one of the following ionic species has the greatest proton affinity to form stable compound?
The stability of + 1 oxidation state increases in the sequence: [2009]
Amongst the elements with following electronic configurations, which one of them may have the highest ionization energy? [2009]
The correct order of the decreasing ionic radii among the following isoelectronic species are :
|
129 videos|244 docs|88 tests
|