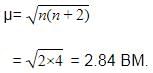Test: JEE Previous Year Questions- Coordination Compounds - NEET MCQ
22 Questions MCQ Test Inorganic Chemistry for NEET - Test: JEE Previous Year Questions- Coordination Compounds
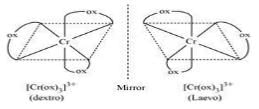
In [Cr(C2O4)3]3_ , the isomerism shown is
[AIEEE-2002]
In the complexes [Fe(H2O)6]3+, [Fe(CN)6]3+, [Fe(C2O4)3]3_ and [FeCl6]3_, more stability is shown by -
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
One mole of the complex compound Co(NH3)5Cl3, gives 3 moles of ions on dissolution in water. One mole of the same complex reacts with two moles of AgNO3solution to yield two moles of AgCl(s). The structure of the complex is –
[AIEEE-2003]
In the coordination compound K4[Ni(CN)4], the oxidation state of nickel is –
[AIEEE-2003]
The number of 3d-electrons remained in Fe2+ (At. no. of Fe = 26) is –
[AIEEE-2003]
Ammonia forms the complex ion [Cu(NH3)4]2+ ion with copper ions in alkaline solutions but not in acidic solution. What is the reason for it –
[AIEEE-2003]
Among the properties (a) reducing (b) oxidising (c) complexing, the set of properties shown by CN_ ion towards metal species is –
[AIEEE-2004]
The coordination number of a central metal atom in a complex is determined by –
[AIEEE-2004]
Which one of the following complexes in an outer orbitals complex–
[AIEEE-2004]
Coordination compounds have great importance in biological systems. In this contect which of the following statements is incorrect ?
[AIEEE-2004]
The correct order of magnetic moments (spin only values in B.M. among is) –
[AIEEE-2004]
The value of the 'spin only' magnetic moment for one of the following configurations is 2.84 BM . The correct one is –
[AIEEE-2005]
The IUPAC name for the complex [Co(NO2)(NH3)5] Cl2 is –
[AIEEE-2006]
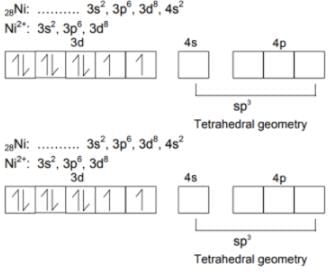
Nickel (Z = 28) combines with a ninegative monodentate ligand X_ to form a paramagnetic complex [NiX4]2_. The number of unpaired electron in the nickel and geometry of this complex ion are respectively –
[AIEEE-2006]
In Fe(CO)5, the Fe–C bond possesses –
[AIEEE-2006]
How many EDTA (ethylenediaminetetraacetate ion) molecules are required to make an octahedral complex with a Ca2+ ion ?
[AIEEE-2006]
The ''spin-only'' magnetic moment [in units of Bohr magneton] of Ni2+ in aqueous solution would be (At. No. Ni = 28) –
[AIEEE-2006]
Which one of the following has a square planar geometry - (Co = 27, Ni = 28, Fe = 28, Fe = 26, Pt = 78) –
[AIEEE-2007]
The coordination number and the oxidation state of the element 'E' in the complex [E(en)2(C2O4)] NO2 (where (en) is ethylene diamine) are, respectively –
[AIEEE-2008]
In which of the following octahedral complexes of Co (at. no. 27), will the magnitude of D0 be the highest ?
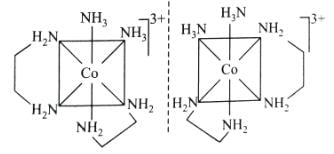
Which of the following has an optical isomer ?
[AIEEE-2009]
Which of the following pairs represents linkage isomers ?
[AIEEE-2009]
|
74 videos|106 docs|111 tests
|
|
74 videos|106 docs|111 tests
|


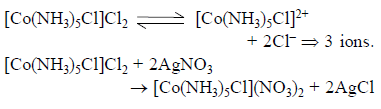
 Thus correct option is A
Thus correct option is A