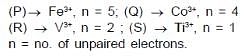Test: Coordination Compounds - 3 - NEET MCQ
20 Questions MCQ Test Topic-wise MCQ Tests for NEET - Test: Coordination Compounds - 3
Which of the following species is not expected to be a ligand?
A complex cation is formed by Pt(in some oxidation state) with ligands (in proper number so that coordination number of Pt becomes six). Which of the following can be its correct IUPAC name?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The EAN of metal atoms in [Fe(CO2)(NO+)2] and Co2(CO8) respectively are:
Match the complex ions listed in column-I with the characteristics listed in column-II using the codes given below.

Which of the following statements is correct?
Note : NO is three electrons donor
All the following complex ions are found to be paramagnetic
P : [FeF6]3- ; Q : [COF6] 3- ; R : [V(H2O)6] 3+ ; S : [Ti(H2O)6]3+
The correct order of their paramagnetic moment (spin only) is
Among [NiCl2 (PPh3)2], [Ni(HDMG)2] and [Ni(CO)4] complexes the hybridisation statesof Ni atom are respectively.
All the following complexes show decrease in their weights when placed in a magnetic
balance then the group of complexes having tetrahedral geometry is:
(i) Ni(CO)4
(ii) K[AgF4]
(iii) Na2[Zn(CN)4]
(iv) K2[PlCl4]
( v )[RhCl(PPh3)3]
The ‘brown ring’ formed at the junction of two layers in the test of nitrate is due to the formation of a complex ion, [Fe(H2O)5NO]2+. Which of the following statements are correct for this complex [u = 3.87 B.M.)
(i) Oxidation state of Fe is +1 and NO exists as NO+.
(ii) The complex ion is in octahedral geometry as attained by sp3d2 hybridisation.
(iii) The complex is paramagnetic and has three unpaired electrons due to transfer of electron from NO to Fe2+.
(iv) The complex is in octahedral geometry as attained by d2sp3 hybridisation.
(v) The brown colour of the complex is attributed to d-d transition of electron.
Which of the following statements is most likely to be incorrect?
Identify the correct order of wavelength of light absorbed for the following complex ions.


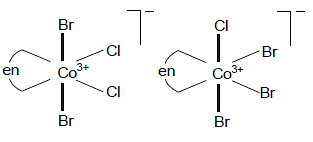
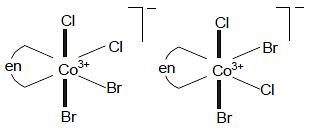
The possible geometric isomers shown by [Co(en)Br2Cl2]- is:
The total number of isomers of the complex, [Co(NH3)4(NO2) 2(NO3) complex is :
Which of the following has both face-mer and optical isomers?
Statement–1 : In complex [Cr(NH3)4 BrCl]Cl, the ‘spin only’ magnetic moment is close to 1.73 B.M.
Statement–2 : All known mononuclear complexes of chromium(III), irrespective of the strength of the ligand field, must have three unpaired electrons.
Statement–1 : The magnitude of for a given ligand increases as the charge on he metal ion increases.
Statement–2 : NH3 is a weak field ligand towards Co2+ but acts as a strong field ligand towards Co3+.
Statement–1 : tris (ethane-1, 2 – diamine)nickel(II) chloride is homoleptic outer orbital complex
Statement–2 : The geometry of Ni in tris (ethane-1, 2 – diamine)nickel(II) chloride is trigonal bipyramidal
Statement–1 : [Ni (H2O)6]2+ is green but a solution of [Ni(CN)4]2- is colourless.
Statement–2 : Energy difference between d levels (i.e. ) for H2 O complex (paramagnetic) is in the visible region and that for the cyano complex (diamagnetic) is in the UV region
|
9 docs|1272 tests
|