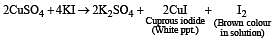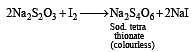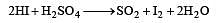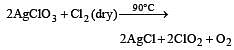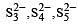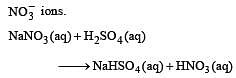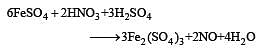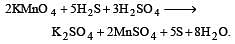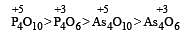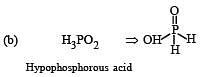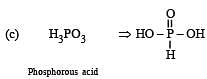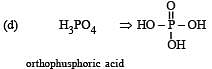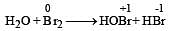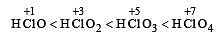31 Year NEET Previous Year Questions: The p-Block Elements - 2 - NEET MCQ
30 Questions MCQ Test Chemistry Class 12 - 31 Year NEET Previous Year Questions: The p-Block Elements - 2
Which of the following fertilizers has the highest nitrogen percentage ? [1993]
A solution of potassium bromide is treated with each of the following. Which one would liberate bromine ? [1993]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
A certain compound (X) when treated with copper sulphate solution yields a brown precipitate. On adding hypo solution, the precipitate turns white.The compound is
Noble gases do not react with other elements because [1994]
Which of the following statements is false ? [1994]
HI can be prepared by all the following methods, except[1994]
Which among the following is paramagnetic ? [1994]
Which one of the following oxides of chlorine is obtained by passing dry chlorine over silver chlorate at 90°C ? [1994]
The formula for calcium chlorite is [1994]
Polyanion formation is maximum in [1994]
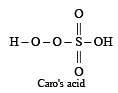
The acid which has a peroxy linkage is [1994]
Brown ring test is used to detect [1994]
About 20 km above the earth, there is an ozone layer. Which one of the following statements about ozone and ozone layer is true? [1995]
By passing H2S gas in acidified KMnO4 solution, we get [1995]
Regarding F– and Cl– which of the following statements is/are correct? [1996]
(i) Cl– can give up an electron more easily than F –
(ii) Cl– is a better reducing agent than F–
(iii) Cl– is smaller in size than F–
(iv)F– can be oxidized more readily than Cl–
Which of the following oxides will be the least acidic?[1996]
Oxidation of thiosulphate by iodine gives [1996]
Which one is the correct order of the size of iodine species? [1997]
Which of the following species has the highest dipole moment ? [1997]
The structural formula of hypophosphorous acid is
A one litre flask is full of brown bromine vapour.The intensity of brown colour of vapour will not decrease appreciably on adding to the flask some[1998]
Repeated use of which one of the following fertilizers would increase the acidity of the soil?
During its reactions, ozone [1999]
Which of the following oxy-acids has the maximum number of hydrogens directly attached to phosphorus? [1999]
Which one of the following arrangements does not truly represent the property indicated against it?[2000]
Nitrogen forms N2, but phosphorus is converted into P4 from P, the reason is [2001]
Which of the following statements is not true ? [2003]
Which is the best description of the behaviour of bromine in the reaction given below ? [2004] H2O + Br2 → HOBr + HBr
The correct order of acid strength is: [2005]
Which of the following is the most basic oxide?
|
108 videos|287 docs|123 tests
|