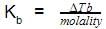Test: Colligative Properties - NEET MCQ
15 Questions MCQ Test Chemistry Class 12 - Test: Colligative Properties
Which one of the following is not a colligative property?
The molal elevation constant is the elevation in boiling point of
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The information that is/are needed to determine the molar mass of an unknown solute is/are
What is the boiling point of a 3 molal aqueous solution if Kb is 0.512°C/m?
A solution was made by dissolving 2 g of a solute in 100 g of acetone. The solution boiled at 56.95° C. The boiling point of pure acetone is 55.95° C, and the Kb =1.71°C/m. What is the molecular weight of the solute?
The molal elevation constant is the ratio of the elevation in B.P to:
Which of the two has lower freezing point, 2m NaCl or 5m NaCl aqueous solution?
If the vapour pressure of pure solvent A is 17.5 mm and lowering of vapour pressure of solution formed by adding a non-volatile electrolyte is 0.0175 mm then what is the relative lowering of vapour pressure?
Which of the following unit of concentration is independent of temperature?
Relative lowering of vapour pressure is directly proportional to
Camphor is used as solvent to determine the molecular mass of non-volatile solute by Rast method because for camphor
|
108 videos|286 docs|123 tests
|