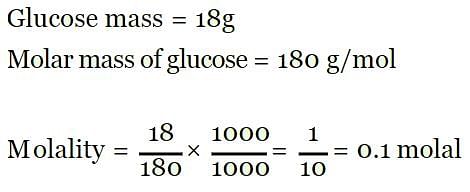Test: Concentration, Empirical & Molecular Formulae - NEET MCQ
17 Questions MCQ Test Chemistry Class 11 - Test: Concentration, Empirical & Molecular Formulae
For the reaction 2x + 3y + 4z → 5w
Initially if 1 mole of x, 3 mole of y and 4 mole of z is taken. If 1.25 mole of w is obtained then % yield of this reaction is
Molarity of NaOH in a solution prepared by dissolving 4 g of NaOH in enough water to form 250 ml of solution is:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
125 ml of 8% w/w NaOH solution (sp. gravity 1) is added to 125 ml of 10% w/v HCl solution. The nature of resultant solution would be ____
Ratio of masses of H2SO4 and Al2 (SO4)3 is grams each containing 32 grams of S is _____
18 g of glucose (C6H12O6) is present in 1000 g of an aqueous solution of glucose. The molality of this solution is:
For the reaction 2A + 3B + 5C → 3D
Initially if 2 mole of A, 4 mole of B and 6 mole of C is taken, With 25% yield, moles of D which can be produced are _____________.
Two elements X (atomic mass = 75) and Y (atomic mass = 16) combine to give a compound having 75.8% of X. The formula of the compound is:
Equal volumes of 10% (v/v) of HCl is mixed with 10% (v/v) NaOH solution. If density of pure NaOH is 1.5 times that of pure HCl then the resultant solution be :
Similar to the % labelling of oleum, a mixture of H3PO4 and P4O10 is labelled as (100 + x) % where x is the maximum mass of water which can react with P4O10 present in 100 gm mixture of H3PO4 and P4O10. If such a mixture is labelled as 127% Mass of P4O10 is 100 gm of mixture, is
C6H5OH(g) + O2(g) → CO2(g) + H2O(l)
The magnitude of volume change if 30 ml of C6H5OH (g) is burnt with an excess amount of oxygen, is
How many moles of NaCl should be dissolved in 100 mL of water to get 0.2 M solution?
If 50 gm oleum sample rated as 118% is mixed with 18 gm water, then the correct option is
The minimum mass of mixture of A2 and B4 required to produce at least 1 kg of each product is:
(Given At. mass of ‘A’ = 10; At mass of ‘B’ = 120) 5A2 + 2B4 → 2AB2 + 4A2B
The % by volume of C4H10 in a gaseous mixture of C4H10, CH4 and CO is 40. When 200 ml of the mixture is burnt in excess of O2. Find volume (in ml) of CO2 produced.
74 gm of sample on complete combustion gives 132 gm CO2 and 54 gm of H2O. The molecular formula of the compound may be
Density of a gas relative to air is 1.17. Find the mol. mass of the gas. [Mair = 29 g/mol]
Number of moles of hydroxide ions in 0.3L of 0.005M solution of Ba (OH)2 is:
|
127 videos|244 docs|87 tests
|