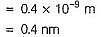Test: Dual Nature of Matter & Heisenberg's Uncertainty Principle - NEET MCQ
16 Questions MCQ Test Chemistry Class 11 - Test: Dual Nature of Matter & Heisenberg's Uncertainty Principle
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
Which of the following properties of atom could be explained correctly by Thomson Model of atom?
The threshold frequency v0 for a metal is 7.0 x 1014 s-1. Radiation of frequency v = 1.0 x 1015 s-1 hits the metal. Kinetic energy of the emitted electron is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following rules/principles is responsible to rule out the existence of definite paths or trajectories of electrons?
In an atom, an electron is moving with a speed of 600 ms-1 with an accuracy of 0.005%. Certainty with which the position of the electron can be located is
[AIEEE 2009]
The wavelength (in nanometer) associated with a proton (mass = 1.67 x 10-27 kg atom-1) at the velocity of 1.0 x 103 ms-1 is
Statement I : It is impossible to determine position and momentum of the moving electron simultaneously with same accuracy.
Statement II : The path of an electron in an atom is clearly defined.
If the photon of the wavelength 150 pm strikes an atom and one of its inner bound electrons is ejected out with a velocity of 1.5 x 107 ms-1, then binding energy by which electron is bound to nucleus is
The position of both, an electron and a helium atom is known within 1.0 mm. Further more the momentum of the electron is known within 5.0 x 10-26 kg ms-1. The minimum uncertainty in the measurement of the momentum of the helium atom is
The wavelength of a neutron with a translatory kinetic energy equal to kT at 300 K is
Frequency of a matter wave is equal to
Direction (Q. Nos. 11 and 12) This section contains a paragraph, wach describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given ptions (a),(b),(c),(d)
Photoelectric effect can be expressed in terms of the following graph
Q. What is work function for this photoelectric emission of electrons?
Photoelectric effect can be expressed in terms of the following graph
Q. Kinetic energy imparted to the moving electron at a wavelength of 2.5 x 10-7 m is
Direction (Q. Nos. 13) Choice the correct combination of elements and column I and coloumn II are given as option (a), (b), (c) and (d), out of which ONE option is correct.
Q.
Neon gas is generally used in sign boards. It emits radiations at wavelength 616 nm.
Match the values derived given in Column II with the corresponding parameter given in Column I.
Direction (Q. Nos. 14 - 16) This section contains 2 questions. when worked out will result in an integer from 0 to 9 (both inclusive)
Q.
The work function () of some metals is listed below. The number of metals which will show photoelectric effect when light of 300 nm wavelength falls on the metal i s ........
If the uncertainties in the measurements of position and momentum are equal, calculate the uncertainty in the measurement of velocity (in ms-1) of particle of mass 1.21 x 10-18 kg
Wavelength associated with an electron having KE 3.0 x 10-25 J is x *10-7 m.
Q. What is the value of x?
|
129 videos|232 docs|88 tests
|