Test: Electrical Properties of Solids (Old NCERT) - NEET MCQ
10 Questions MCQ Test Chemistry Class 12 - Test: Electrical Properties of Solids (Old NCERT)
Which of the following shows correct range of conductivity?
(i) Conductors: 104 to 107 ohm−1 m−1
(ii) Insulators: 10−6 to 104 ohm−1 m-1
(iii) Semiconductors: : 10−10 to 10−6 ohm−1 m−1
(i) Conductors: 104 to 107 ohm−1 m−1
(ii) Insulators: 10−6 to 104 ohm−1 m-1
(iii) Semiconductors: : 10−10 to 10−6 ohm−1 m−1
Pure silicon and germanium behave as
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
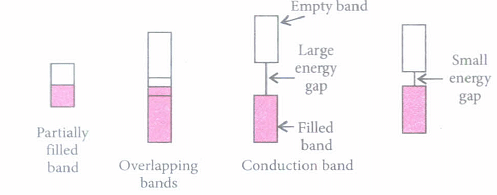
Three type of bands are shown in the figures given below showing the position of the valence band and conduction band. The figures A,B and C represent
The conductivity of intrinsic semiconductors can be increased by adding a suitable impurity. This process is called (P). This can be done with an impurity which is (Q) rich or deficient as compared to the semiconductor. Such impurities introduce (R) defects in them. Electron rich impurities result in (S) type semiconductors while electron deficit impurities result in (T) type semiconductors.

To get n-type of semiconductor, germanium should be doped with
p−type semiconductors are formed when Si or Ge are doped with:
Which type of semiconductor is formed when grmanium is doped in the gallium as indicated in the figure?

Which of the following statements is true about semiconductors?
Observe the given figure carefully and fill in the blanks by choosing the correct option.

Match the column I with column II and mark the appropriate choice.

|
103 videos|282 docs|123 tests
|


















