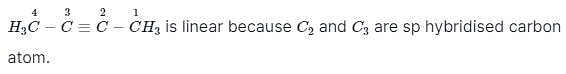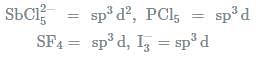Test: Hybridisation - NEET MCQ
22 Questions MCQ Test Chemistry Class 11 - Test: Hybridisation
Direction (Q. Nos. 1-11) This section contains 11 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE option is correct.
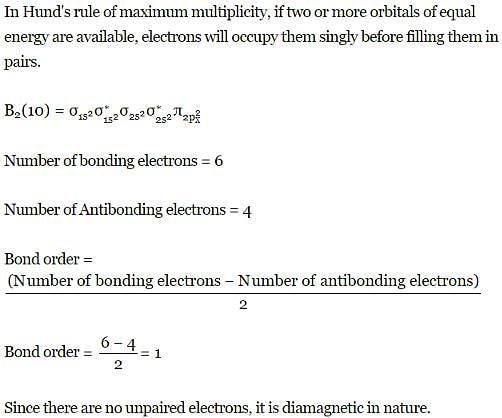
Q. Assuming that Hund's rule is violated, the bond order and magnetic nature of the diatomic molecule B2 is
Which of the following statement is incorrect regarding NO2 molecule?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Molecule MX3 (atomic number M < 21) has zero dipole moment, the sigma bonding orbitals used by M are
bond in between (C2— C3) vinyl acetylene is formed by ...... overlapping
Hydrogen bonds are formed in many compounds e.g., H2O, HF, NH3 . The boiling point of such compounds depends to a large extent on the strength of hydrogen bond and the number of hydrogen bonds. The correct decreasing order of the boiling points of above compounds is :
Considering the state of hybridisation of C-atoms,select the molecule among the following which is linear
In which one of the following species the central atom has the type of hybridisation which si not the same as that present in the other three?
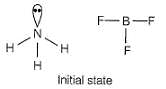
Specify the coordination geometry and hybridisation of N and B atoms in a 1:1 complex of BF3 and NH3.
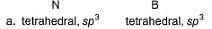

If there are five electron pairs in outer shell, then structure and bond angle as predicted by Sidgwick-Powell theory is
Direction (Q. Nos. 12 and 14) This sectionis based on statement I and Statement II. Select the correct answer from the code given below.
Q.
Statement I : BF3 molecule is planar but NF3 is pyramidal.
Statement II : N atom is smaller than B.
Statement I : B atom is sp2-hybridised in B2H6.
Statement II : There is no lone pair or unpaired electron in B2H6.
Statement I : BCI3 exists as monomer while AICI3 exists as dimer.
Statement II : Boron atom is too small to form the stable 4-membered ring system
Direction (Q. Nos. 15-17) This section contains 3 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q.
Select the correct order of bond lengths of the specified bonds.
Direction (Q. Nos. 18-19) This section contains a paragraph, each describing theory, experiments, data etc. three Questions related to paragraph have been given.Each question have only one correct answer among the four given options (a),(b),(c),(d).
In certain polar solvents, PCI5 undergoes an ionisation reaction as shown
Q. Select the incorrect statement (s).
In certain polar solvents, PCI5 undergoes an ionisation reaction as shown
Q. Number of lone pairs on P in I, il and III are
Direction (Q. Nos. 20-21) This section contains 4 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THANT ONE is correct.
Q. Match the following compounds given in Column I with their geometry given is Column II
Match the processes in Column I with type of changes in hybridisation in Column II.

Direction (Q. Nos. 22) This section contains 1 questions. when worked out will result in an integer from 0 to 9 (both inclusive)
Q. Most acidic H in the following compound is attached to C-atoms denoted by at S-N. ( ) ...
|
127 videos|232 docs|88 tests
|