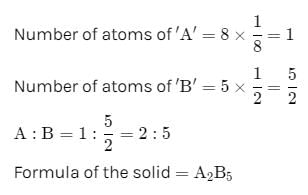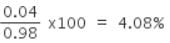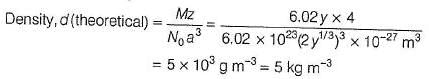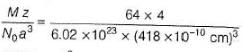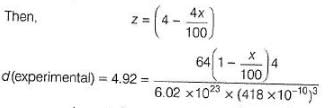Test: Imperfections in Solids (Old NCERT) - NEET MCQ
21 Questions MCQ Test Physical Chemistry for NEET - Test: Imperfections in Solids (Old NCERT)
Only One Option Correct Type
This section contains 9 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q.
This section contains 9 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
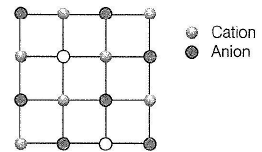
Structure shown in the figure represents
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following defects is also known as dislocation defect?
Cations are present in the interstitial sites in
Select the correct statement about non-stoichiometric compounds
In a face-centred cubic lattice, atom A occupies the corners of the cube and atom B occupies the face-centred positions. If one atom of B is missing from one of the face-centred points, the formula of the compound is
An element crystallises in fcc lattice having edge length 40 0 pm. Maximum radius of the atom which can be placed in the interstitial site without distorting the structure is
Experimentally it was found that a metal oxide has formula M0.98O. Metal M, is present as M2+ and M3+ in its oxide. The fraction of the metal which exists as M3+ would be:
If NaCI is doped with 10-3 mole % of SrCI2 then , number of cation ic vacancies is
One or More than One Options Correct Type
This section contains 7 multiple type questions. Each question has 4 choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
AgBr(s) crystals can exhibit one or more types of defect which are
Substitution of foreign atom in the site of parent atom in crystal is a?
As grain size of metal increases, Strength of metal:
Select the correct statements about the given structure.
Select the correct statement(s) about following structure.
Which of the following statement(s) is/are correct?
Comprehension Type
This section contains a passage describing theory, experiments, data, etc. Two questions related to the paragraph have been given. Each question has only one correct answer out of the given 4 options (a), (b), (c) and (d).
Passage I
The crystal AB (rock salt structure) has m ole cular w eight 6.02 y u, where y is an arbitrary unit in u. Also, given minimum distance between cation and anion = y 1/3 nm and observed density = 20 kg m-3.
Q.
Theoretical density of AB is
Passage
The crystal AB (rock salt structure) has m ole cular w eight 6.02 y u, where y is an arbitrary unit in u. Also, given minimum distance between cation and anion = y 1/3 nm and observed density = 20 kg m-3.
Q.
Defect present in the crystal is of the type
Passage II
Titanium (II) oxide has a rock salt structure. X-ray diffraction data shows that the length of one edge of the cubic unit cell for TiO with 1:1 ratio of Ti to O is 418 pm and the density is 4.92 g cm-3. ( Atomic mass of Ti = 48).
Q.
Defect present in the crystal is of the type
Passage II
Titanium (II) oxide has a rock salt structure. X-ray diffraction data shows that the length of one edge of the cubic unit cell for TiO with 1:1 ratio of Ti to O is 418 pm and the density is 4.92 g cm-3. ( Atomic mass of Ti = 48).
Q.
% of vacancies (defects) in the structure is
Matching List Type
Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
One Integer Value Correct Type
This section contains 1 question, when worked out will result in an integer value from 0 to 9 (both inclusive)
Q.
Calcium crystallises in a face-centred cubic unit cell with a = 0.556 nm and density 1.4848 g/cm3. Percentage of Schottky defects in this crystal is ... .
|
117 videos|225 docs|239 tests
|
|
117 videos|225 docs|239 tests
|