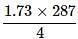Test: Packing Efficiency (Old NCERT) - NEET MCQ
10 Questions MCQ Test Chemistry Class 12 - Test: Packing Efficiency (Old NCERT)
The edge length of a fcc is 508 pm. If the radius of cation is 110 pm, the radius of anion is
A metal X crystallises in a face-centred cubic arrangement with the edge length 862 pm. What is the shortest separation of any two nuclei of the atom ?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The edge length of sodium chloride unit cell is 564 pm. If the size of Cl- ion is 181 pm. The size of Na+ ion will be
If the distance between Na+ and Cl- ions in NaCl crystals is 265 pm, then edge length of the unit cell will be?
The radius of Na+ is 95pm and that of Cl- is 181 pm. The edge length of unit cell in NaCl would be (pm).
Copper crystallises in fcc with a unit cell length of 361 pm. What is the radius of copper atom?157 pm
Total volume of atoms present in a fcc unit cell of a metal with radius r is
The relation between atomic radius and edge length 'a' of a body centred cubic unit cell:
Edge length of unit cell of Chromium metal is 287 pm with the arrangement. The atomic radius is the order of:
The fraction of total volume occupied by the atoms present in a simple cube is
|
100 videos|282 docs|123 tests
|




 ∴ d = √3a / 2
∴ d = √3a / 2