NEET Exam > NEET Tests > Chemistry Class 12 > Test: Rate of a Chemical Reaction - NEET MCQ
Test: Rate of a Chemical Reaction - NEET MCQ
Test Description
10 Questions MCQ Test Chemistry Class 12 - Test: Rate of a Chemical Reaction
Test: Rate of a Chemical Reaction for NEET 2024 is part of Chemistry Class 12 preparation. The Test: Rate of a Chemical Reaction questions and answers have been
prepared according to the NEET exam syllabus.The Test: Rate of a Chemical Reaction MCQs are made for NEET 2024 Exam. Find important
definitions, questions, notes, meanings, examples, exercises, MCQs and online tests for Test: Rate of a Chemical Reaction below.
Solutions of Test: Rate of a Chemical Reaction questions in English are available as part of our Chemistry Class 12 for NEET & Test: Rate of a Chemical Reaction solutions in
Hindi for Chemistry Class 12 course. Download more important topics, notes, lectures and mock
test series for NEET Exam by signing up for free. Attempt Test: Rate of a Chemical Reaction | 10 questions in 15 minutes | Mock test for NEET preparation | Free important questions MCQ to study Chemistry Class 12 for NEET Exam | Download free PDF with solutions
Test: Rate of a Chemical Reaction - Question 1
A foreign substance that increase the speed of a chemical reaction is called
Detailed Solution for Test: Rate of a Chemical Reaction - Question 1
Test: Rate of a Chemical Reaction - Question 2
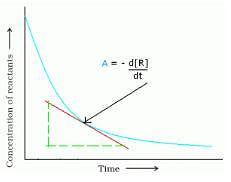
. What will be the value of instantaneous rate of reaction from the graph?

Detailed Solution for Test: Rate of a Chemical Reaction - Question 2
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Test: Rate of a Chemical Reaction - Question 3
The rate of a chemical reaction doubles for every 10°C rise of temperature. If the temperature is raised by 50°C, the rate of the reaction increases by about
[AIEEE 2011]
Detailed Solution for Test: Rate of a Chemical Reaction - Question 3
Test: Rate of a Chemical Reaction - Question 4
Chemical substances speeding up rate of chemical reaction is called as
Detailed Solution for Test: Rate of a Chemical Reaction - Question 4
Test: Rate of a Chemical Reaction - Question 5
Chemical substances speeding up the rate of chemical reaction is called as
Detailed Solution for Test: Rate of a Chemical Reaction - Question 5
Test: Rate of a Chemical Reaction - Question 6
The rate constant of zero-order reactions has the unit
Detailed Solution for Test: Rate of a Chemical Reaction - Question 6
Test: Rate of a Chemical Reaction - Question 7
For the reaction A + B → C+ D . The variation of the concentration of the products is given by curve
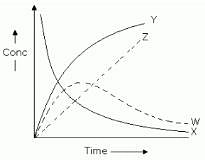
Detailed Solution for Test: Rate of a Chemical Reaction - Question 7
Test: Rate of a Chemical Reaction - Question 8
The term -dx/dt in the rate expression refers to the:
Detailed Solution for Test: Rate of a Chemical Reaction - Question 8
Detailed Solution for Test: Rate of a Chemical Reaction - Question 9
Detailed Solution for Test: Rate of a Chemical Reaction - Question 10
|
102 videos|282 docs|123 tests
|
Information about Test: Rate of a Chemical Reaction Page
In this test you can find the Exam questions for Test: Rate of a Chemical Reaction solved & explained in the simplest way possible.
Besides giving Questions and answers for Test: Rate of a Chemical Reaction, EduRev gives you an ample number of Online tests for practice


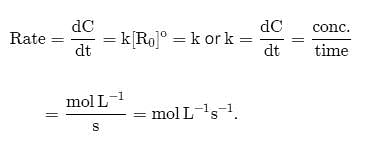
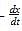 in the rate expression refers to the instantaneous rate of the reaction.
in the rate expression refers to the instantaneous rate of the reaction.