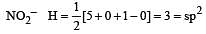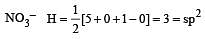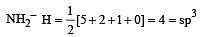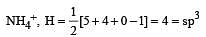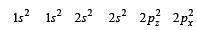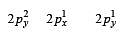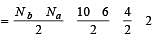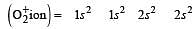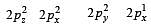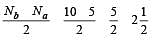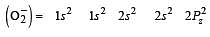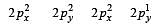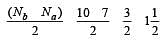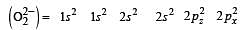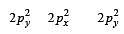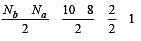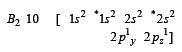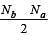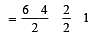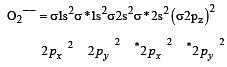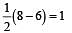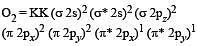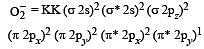31 Year NEET Previous Year Questions: Chemical Bonding & Molecular Structure - 1 - NEET MCQ
30 Questions MCQ Test Chemistry Class 11 - 31 Year NEET Previous Year Questions: Chemical Bonding & Molecular Structure - 1
In an octahedral structure, the pair of d orbitals involved in d2sp3 hybridization is [2004]
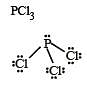
In BrF3 molecule, the lone pairs occupy equatorial positions to minimize [2004]
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Which of the following is the electron deficient molecule? [2005]
The correct sequence of increasing covalent character is represented by [2005]
Which of the following molecules has trigonal planar geometry? [2005]
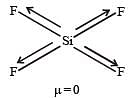
Which of the following would have a permanent dipolemoment? [2005]
Which of the following is not a correct statement?
The number of unpaired electrons in a paramagnetic diatomic molecule of an element with atomic number 16 is [2006]
In which of the following molecules all the bonds are not equal? [2006]
The electronegativity difference between N and F is greater than that between N and H yet the dipole moment of NH3 (1.5 D) is larger than that of NF3 (0.2D). This is because [2006]
Which of the following species has a linear shape ?
Which of the following is not isostructural with SiCl4?[2006]
In which of the following pairs, the two species are iso-structure?
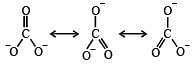
The correct order of C–O bond length among CO, CO32- , CO2 is [2007]
The angular shape of ozone molecule (O3) consists of : [2008]
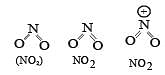
The correct order of increasing bond angles in the following triatomic species is : [2008]
Four diatomic species are listed below in different sequences. Which of these presents the correct order of their increasing bond order ? [2008]
What is the dominant intermolecular force or bond that must be overcome in converting liquid CH3OH to a gas? [2009]
In which of the following molecules / ions BF3, NO2-, NH2- and H2O , [2009] the central atom is sp2 hybridized ?
In which of the following pairs of molecules/ ions, the central atoms have sp2 hybridization? [2010]
In which one of the following species the central atom has the type of hybridization which is not the same as that present in the other three?
Some of the properties of the two species, NO3- and H3O+ are described below. Which one of them is correct? [2010]
In which of the following molecules the central atom does not have sp3 hybridization? [2010]
Considering the state of hybridization of carbon atoms, find out the molecule among the following which is linear ? [2011]
Which of the two ions from the list given below that have the geometry that is explained by the same hybridization of orbitals, NO2–, NO3–, NH2–, NH4+, SCN– ? [2011]
Which one of the following pairs is isostructural (i.e., having the same shape and hybridization)? [2012]
Bond order of 1.5 is shown by : [2012]
Which of the following species contains three bond pairs and one lone pair around the central atom ?[2012]
The pair of species with the same bond order is : [2012]
During change of O2 to O2- ion, the electron adds on which one of the following orbitals ? [2012 M]
|
127 videos|232 docs|88 tests
|


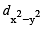 and
and 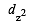 orbitals.
orbitals.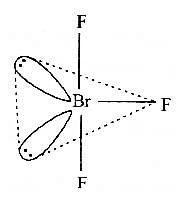
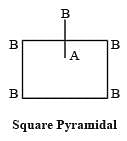
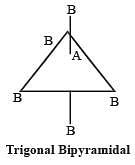
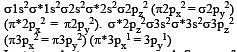
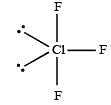
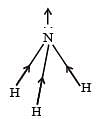
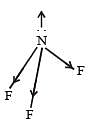
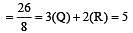
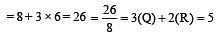
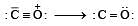
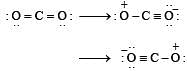
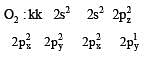

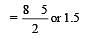
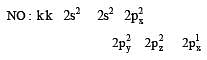
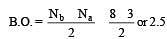
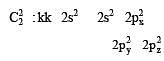
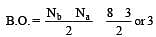

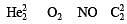
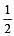 [Group number of central atom –Valency of the central atom].
[Group number of central atom –Valency of the central atom].
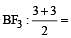 3 means sp2
3 means sp2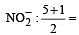 3 means sp2
3 means sp2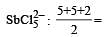 6 means sp3d2
6 means sp3d2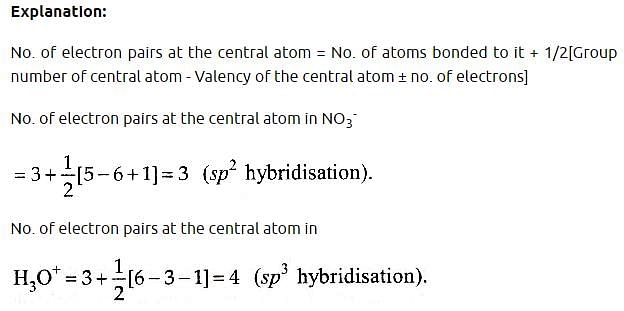
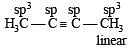
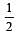 [No. of valenceelectrons of central atom + no. of monovalent atoms attached to it – Negative charge if any – positive charge if any]
[No. of valenceelectrons of central atom + no. of monovalent atoms attached to it – Negative charge if any – positive charge if any]