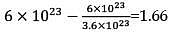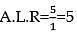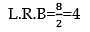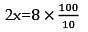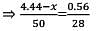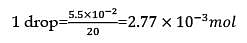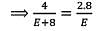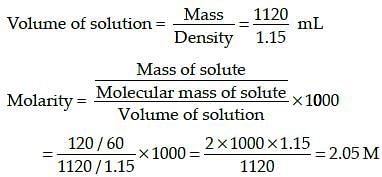NEET Previous Year Questions: Some Basic Concepts Of Chemistry - NEET MCQ
20 Questions MCQ Test Chemistry Class 11 - NEET Previous Year Questions: Some Basic Concepts Of Chemistry
20 ml of decinormal solution of NaOH neutralises 25 ml of a solution of dibasic acid containing 3g. of the acid per 500 ml. The Molecular weight of the acid is
In a compound C, H and N are present in the ratio 9: 1: 3.5 by weight. Molecular mass of the compound is 108. Molecular formula of the compound is:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Number of atoms is 560 g of Fe (atomic mass = 56 g mol-1)
What volume of hydrogen gas at 273 K and 1 atm pressure will be consumed in obtaining 21.6 g elemental boron (atomic mass = 10.8) from the reduction of boron trichloride by hydrogen?
One mole of magnesium nitride on reaction with an excess of water gives
How many moles of magnesium phosphate, Mg3 (PO4)2 will contain 0.25 mole of oxygen atoms?
In the reaction, 2Al (s) + 6HCl (aq) → 2Al3+ (aq) + 6Cl- (aq) + 3H2(g)
Volume occupied by one molecule of water (density = 1 g cm-3) is
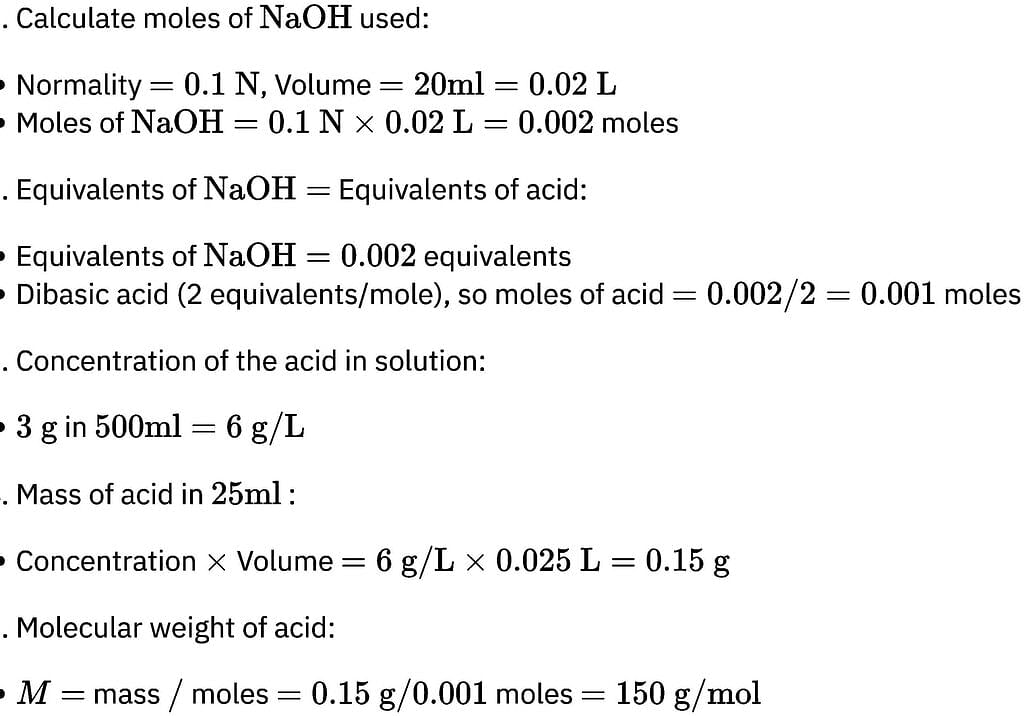
How many moles of lead (II) chloride will be formed from a reaction between 6.5 g of Pbo and 3.2 g of HCl?
100mL of PH3 on heating forms P4 and H2, volume changes in the reaction is:
Excess of carbon dioxide is passed through 50 mL of 0.5 M calcium hydroxide solution. After the composition of the reaction, the solution was evaporated to dryness. The solid calcium carbonate was completely neutralized with 0.1 N hydrochloric acid. The volume of the hydrochloric acid required is___________
25.3 g of sodium carbonate, Na2CO3 is dissolved in enough water to make 250 mL of solution. If sodium carbonate dissociates completely, molar concentration of sodium ions, Na+ and carbonate ions, 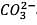 are respectively. (At. mass of carbon = 40)
are respectively. (At. mass of carbon = 40)
The number of atoms in 0.1 mol of a triatomic gas is
How much time (in hours) would it take to distribute one Avogadro number of wheat grains if 1020 grains are distributed each second?
For a reaction A + 2B → C, the amount of C formed by starting the reaction with 5 moles of A and 8 moles of B is
A mixture of CaCl2 and NaCl weighing 4.44 g is treated with sodium carbonate solution to precipitate all the Ca2+ ions as calcium carbonate. The CaCO3 so obtained is heated strongly to get 0.56 g of CaO. The percentage of NaCl in the mixture is
If 1 mL of water contains 20 drops, then number of molecules in a drop of water is
In an experiment, 4 g of M2Ox oxide was reduced to 2.8g of the metal.Tf the atomic mass of the metal is 56 g mol-1, the number of O atoms in the oxide is
Which has the maximum number of molecules among the following?
The density of a solution prepared by dissolving 120 g of urea in 1000 g of water is 1.15 g/ml. The molarity of this solution is:
|
127 videos|232 docs|88 tests
|


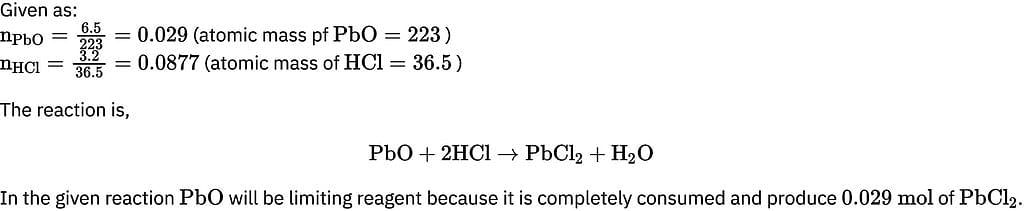
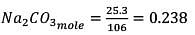
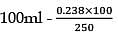
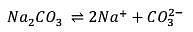
 0.955
0.955