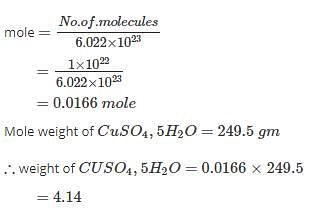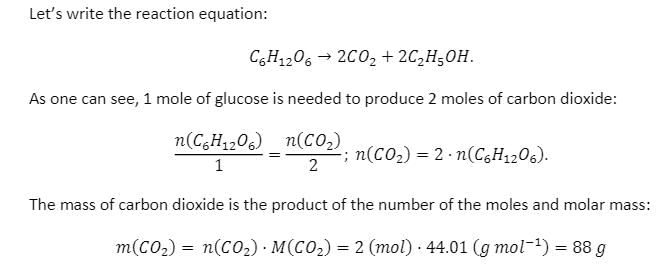RC Mukherjee Test: Mass & Stoichiometry - NEET MCQ
15 Questions MCQ Test Topic-wise MCQ Tests for NEET - RC Mukherjee Test: Mass & Stoichiometry
For the reaction 2NaN3(s) → 2Na(s) + 3N2 (g), how many moles of sodium azide (NaN3) must react in order to produce 8.75 moles of nitrogen gas?
Amount of water produced by the combustion of 16g of methane is:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Branch of chemistry in which quantities of reactants and products of a chemical reaction are determined is known as:
The mass of 1 Χ 1022 molecules of CuSO4.5H2O is:
The mass of one mole of NaOH will be (At. mass of Na = 23, O = 16, H = 1)
If 2 moles of H2 and O2 are available for the reaction, identify the limiting reagent for the reaction,
2H2 + O2 → 2H2O
Which of the following has more number of atoms?
Molecular mass of glucose molecule (C6H12O6) is
If 0.5 mole of BaCl2 is mixed with 0.2 mole of Na3PO4 ,then maximum number of moles of Ba3(PO4)2 that can be formed
The atomic mass of an element is usually fractional because:
If you have 2 moles of A and 3 moles of B, Identify the limiting reagent in the following reaction.
A + B → AB
According to the chemical equation,
Mg(s) + 2HCl → MgCl2(aq) + H2(g)
How many moles of magnesium are needed to produce 12.34 moles of hydrogen gas?
Two different atoms A and B combine together to form AB. What will be AB?
One type of anaerobic respiration converts glucose, C6H12O6, to ethanol C2H5OH and carbon dioxide. If the molecular weight of glucose is 180 grams/mol and the molar mass of ethanol is 50 g/mol how many grams of carbon dioxide are produced when 1 mol of glucose is digested via respiration?
|
9 docs|1272 tests
|