Test: Diazonium Salts - NEET MCQ
20 Questions MCQ Test Chemistry Class 12 - Test: Diazonium Salts
Benzene diazonium chloride when reacts with hypophosphorus acid produces
p-amino azo benzene is obtained by treating diazoniumchloride with:
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
When hypophosphorous acid is treated with diazonium salts, it is reduced to:
Which of the following amine will form stable diazonium salt at 273-283 K ?
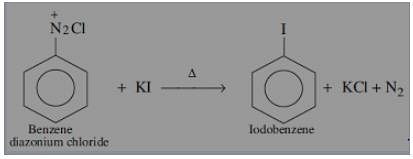
Replacement of diazo group by other groups is helpful in preparing those substituted aromatic compounds which cannot be prepared by
Which of the following amine gives diazonium salt on reaction with HNO2?
On heating an aliphatic primary amine with chloroform and ethanolic potassium hydroxide, the organic compound formed is
What happens when benzene diazonium chloride is treated with potassium cyanide in presence of Cu powder?
When ethanol is treated with benzene diazoniumchloride it forms:
The conversion of primary aromatic amines into diazonium salts is known as:
By treating diazonium salts with cuprous cyanide or KCN and copper powder it forms:
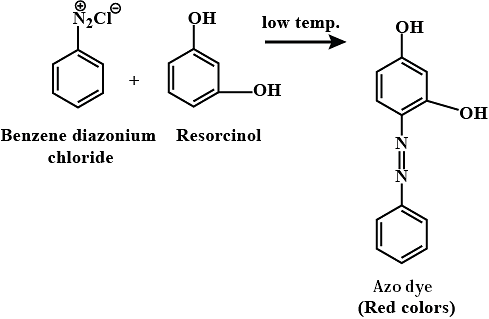
Benzene diazonium chloride on reaction with phenol in weakly basic medium gives:
When diazonium salt solution is treated with water at a temperature of 283 K it forms?
|
100 videos|282 docs|123 tests
|


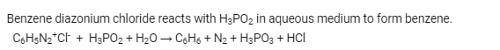
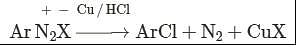
 This reaction is called Gattermann reaction. In this reaction, Cl, Br and CN can be introduced into the benzene ring by simply treating diazonium salts with HCl, HBr, KCN. Respectively in presnce of copper powder instead of using Cu(I) salts.
This reaction is called Gattermann reaction. In this reaction, Cl, Br and CN can be introduced into the benzene ring by simply treating diazonium salts with HCl, HBr, KCN. Respectively in presnce of copper powder instead of using Cu(I) salts.