Test: IUPAC Nomenclature of Coordination Compounds - NEET MCQ
25 Questions MCQ Test Chemistry Class 12 - Test: IUPAC Nomenclature of Coordination Compounds
Direction (Q. Nos. 1-10) This section contains 10 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONLY ONE is correct.
Q. According to IUPAC nomenclature, sodium nitroprusside is named as
The formula of the complex hexamminecobalt (III) chloride sulphate is
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
The IUPAC name of the compound [Cr(NH3)5(NCS)][ZnCI4] is
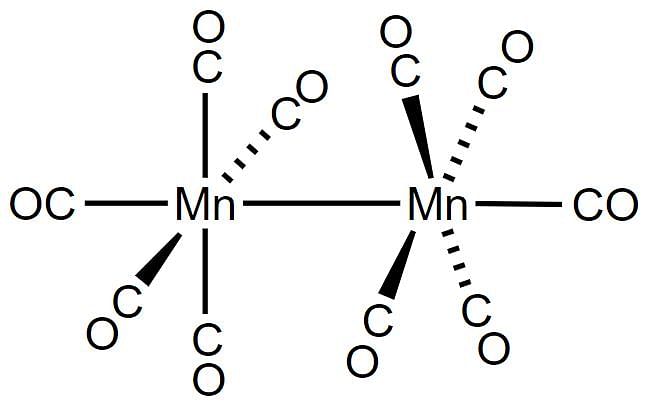
The IUPAC name of the complex [(CO)5Mn - Mn(CO)5] is
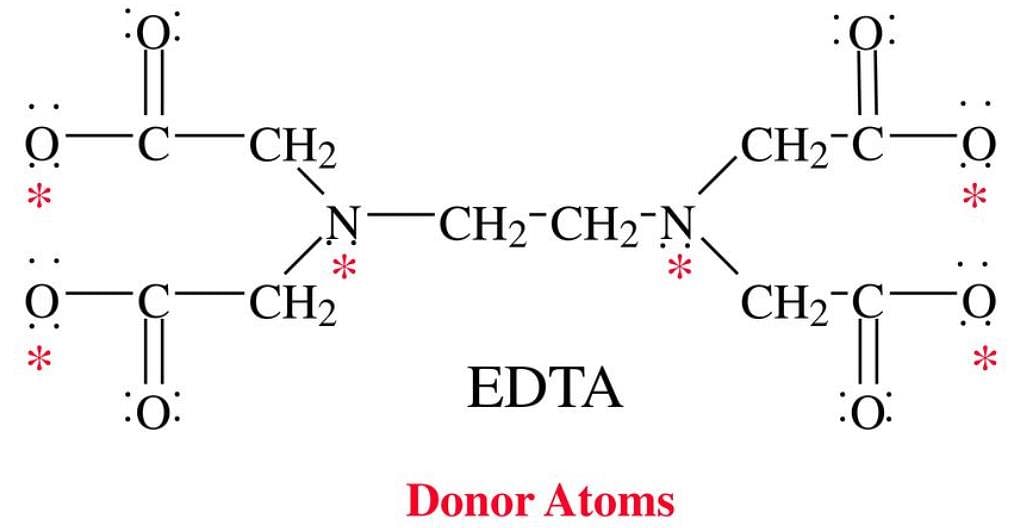
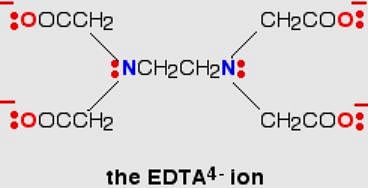
The donor sites of EDTA ligand are
The IUPAC name of [Ni(PPh3)2CI2]2+ is
Formula of brown ring complex is
The IUPAC name of the compound K[SbCl5Ph] is
What is the IUPAC name of compound NaBH4?
Direction (Q. Nos. 11-15) This section contains 5 multiple choice questions. Each question has four choices (a), (b), (c) and (d), out of which ONE or MORE THAN ONE are correct.
Q.
Which of the following complexes have correct name?
Which is/are the correctly named?
The correct name of ligand(s) in IUPAC name is/are
The ionisation isomer of [Cr(H2O)4Cl(NO2)Cl] is
The ligand(s) which gives chelate complexes is/are
Comprehension Type
Direction (Q. Nos. 16 and 17) This section contains a paragraph, describing theory, experiments, data, etc. Two questions have been related to the paragraph have been given. Each question has only one correct answer among the four given options (a), (b), (c) and (d).
Passage
In naming complexes, cation is named first followed by anion. Non-ionic or neutral complexes are given a one word name. The cationic and neutral complex ions are named by writing ‘the number and name of the ligands followed by the name of the central metal ion with its oxidation number in roman numerals within the parenthesis. If the complex is anionic, name of the metals ends with ‘ate’. When the complex contain two or more metal atoms linked with the help of bridging ligand then each bridging ligand is started with μ.
Q.
Name of [CoBr(NH3)4 NO2] is
In naming complexes, cation is named first followed by anion. Non-ionic or neutral complexes are given a one word name. The cationic and neutral complex ions are named by writing ‘the number and name of the ligands followed by the name of the central metal ion with its oxidation number in roman numerals within the parenthesis. If the complex is anionic, name of the metals ends with ‘ate’. When the complex contain two or more metal atoms linked with the help of bridging ligand then each bridging ligand is started with μ.
Q.
The IUPAC name of [Ni(NH3)4] [NiCI4] is
Matching List Type
Direction (Q. Nos. 18 and 19) Choices for the correct combination of elements from Column I and Column II are given as options (a), (b), (c) and (d), out of which one is correct.
Q.
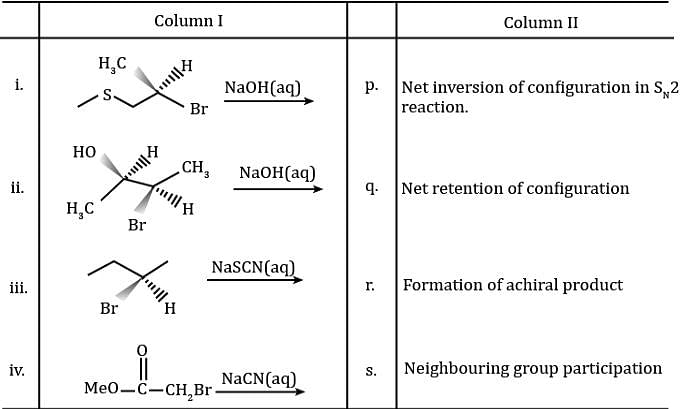
Match the Column I with Column II and mark the correct option from the codes given below.
Match the column I with column II and mark the correct option from the codes given below.
One Integer Value Correct Type
Direction (Q. Nos. 20-24) This section contains 5 questions. When worked out will result in an integer from 0 to 9 (both inclusive).
Q. Ethylenediamminetetraacetate ion is a polydentate ligand and negatively charged. The magnitude of negative charge is
The oxidation state of the central metai in the complex ion [Fe(EDTA)]- .
The complex potassium dicyanodioxalatonickelate (II) in solution produce....... ions.
Number of EDTA molecules required to form an octahedral complex.
IUPAC name of [Pt(NH3)2Cl(NO2)] is
Statement Type
Direction (Q. No. 25) This section is based on Statement I and Statement II. Select the correct answer from the codes given below.
Q.
Statement l : IUPAC name of the complex [(NH3)5—Cr—OH—Cr (NH3)5]CI5 is pentammine chromium-μ-hydroxopentammine chromium (III) chloride.
Statement II : IUPAC name of the complex [Ni(dmg)2] is bis (dimethylglyoximato) nickel (III).
|
100 videos|282 docs|123 tests
|