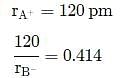Test: Number of Atoms in a Unit Cell & Closed-Packed Structures (Old NCERT) - NEET MCQ
25 Questions MCQ Test Chemistry Class 12 - Test: Number of Atoms in a Unit Cell & Closed-Packed Structures (Old NCERT)
For the structure given below the site marked as S is a

Which of the following statements is not about the voids?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
In ABC packing if the number of atoms in the unit cell is n then the number of tetrahedral voids in the unit cell is equal to:
If the radius of an octahedral void is r and the radius of atoms in close packing is R, then the relation between r and R is :
A metal crystallises into a lattice containing a sequence of layers as AB AB AB ... . What percentage of voids are left in the lattice?
In ccp arrangement the pattern of successive layers can be designated as
Which of the following statements is not correct about hexagonal close packing?
A crystalline structure has radius ratio (r+/r-) in the range of 0.225 - 0.414. The coordination number and arrangement of anions around the cations are:
A Crystal lattice with alternate +ve "and "-veions has radius ratio of 0.524. The coordination number of lattice is
A solid AB has a rock salt structure. If radius of cation A+ is 120 pm, what is the minimum value of radius of B− anion?
A crystal formed by two elements X and Y in cubic structure. X atoms are at the corners of cube while Y atoms are at the face center. The formula of he compound will be:
If three elements X,Y and Z crystallizes in a ccp lattice with the X atoms at the corner, Y atoms at the cube centre and Z atom at the edge centre the formula of the compound will be:
A compound is formed by two elements Y and Z. The element Z forms ccp and atoms Y occupy 1/3rd of tetrahedral voids. The formula of the compound is
A cubic solid is made up of two elements P and Q. Atoms of P are present at the corners of the cube and atoms of Q are present by body centre. What is the formula of the compound and what are coordination number of P and Q?
How many chloride ions are surrounding sodium ion in a sodium chloride crystal?
In NaCl structure,
NaCl type crystal (with coordination no. 6:6) can be converted into CsCl type crystal (with coordination no. 8:8) by applying:
Coordination number of Cs+ and Cl- in CsCl crystal are:
In zinc blende structure,
Consider the unit cell given below :
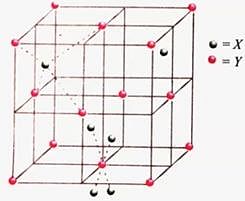
The unit cell shown in the figure belongs to
Consider the unit cell given below :

The coordination number of Y will be
Consider the unit cell given below :

Which of the following about the above structure is not correct?
In CaF2 type (fluorite structure), Ca2+ ions form__(A)___ structure and F ions are present in all __(B)___ voids. The coordination number of Ca2+ is ___(C)___ and F− is___(D)____.
(A), (B), (C) and (D) respectively are:
A unit of BaCl2 (fluorite structure) is made up of:
Which of the following structures is not correctly matched ?
|
102 videos|282 docs|123 tests
|