Lakhmir Singh & Manjit Kaur Test: Matter in Our Surrounding - Class 9 MCQ
25 Questions MCQ Test Science Class 9 - Lakhmir Singh & Manjit Kaur Test: Matter in Our Surrounding
What is the physical state of water at 100°C?
An Almirah is a solid because its
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Find the incorrect statement
Which of the following does not affect rate of evaporation?
Convert 300K into Celsius
Arrange the following substances in increasing order of attraction between the particles: water, sugar, oxygen.
Which of the following will not sublimate ?
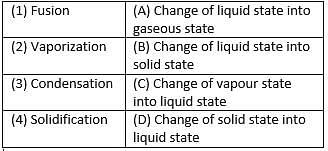
Match the following with correct response.
Which of the following does not affect rate of evaporation?
The interparticle forces are the strongest in
Which one of the following decreases the extent of evaporation of water?
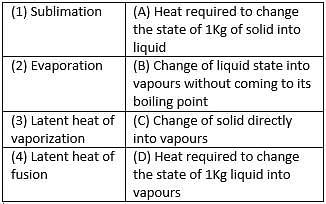
Match the following with correct response.
Name the state of matter that ‘has minimum interparticle attraction’
Pressure on the surface of a gas is increased. What will happen to the inter-particle forces?
A desert cooler given comfort due to cooling caused by evaporation of water. Under which one of the following conditions it works more effectively?
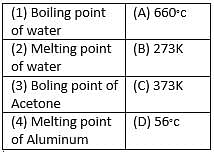
Match the following with correct response.
Identify the incorrect statement
Statement A: A substance is said to be in the liquid state if under normal pressure its M. P. is below the room temperature.
Statement B: The melting point of solid and the freezing point of liquid is different.
Q. Which of the two statements is true?
When alcohol is kept in a china dish it evaporates, temperature falls and cooling is produced. Which one is the correct method to record the temperature?
Identify the incorrect statement about evaporation
A. It causes cooling
B. It increase with increase in humidity
C. It decreases with increase in temperature
D. It increases with increase in wind speed
Statement A: Celsius scale is the best scale for measuring temperature.
Statement B: CO2 is heavier gas than both N2 and O2.
Which of the two statements is true?
Find the incorrect statement
In an endothermic process heat is absorbed and in an exothermic process heat is evolved is observed. What is the nature of evaporation of water?
What is the physical state of water at 10°C?
Which of the following statements is not true?
|
87 videos|369 docs|67 tests
|

















