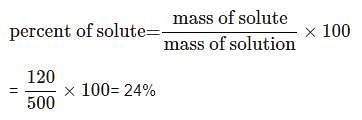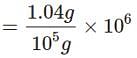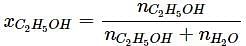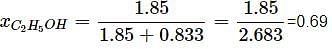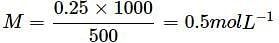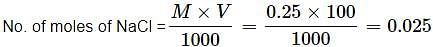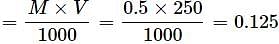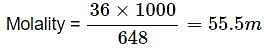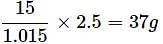Test: Expressing Concentration of Solutions (NCERT) - NEET MCQ
15 Questions MCQ Test Chemistry Class 12 - Test: Expressing Concentration of Solutions (NCERT)
What is the mass percentage of carbon tetrachloride if 22g of benzene is dissolved in 122g of carbon tetrachloride?
What is the mole fraction of glucose in 10% w/W glucose solution?
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Calculate the percentage composition of a solution obtained by mixing 300 g of a 20% and 200 g of a 30% solution by weight.
When 1.04g of BaCl2 is present in 105g of solution, the concentration of solution is:
What will be the mole fraction of ethanol in a sample of spirit containing 85% ethanol by mass?
What is the molarity of a solution containing 10 g of NaOH in 500 mL of solution?
What will be the molarity of 30mL of 0.5M H2SO4 solution diluted to 500mL?
How many Na+ ions are present in 100 mL of 0.25 M of NaCl solution?
How many grams of NaOH are present in 250 mL of 0.5 M NaOH solution?
250mL of sodium carbonate solution contains 2.65g of Na2CO3. If 10mL of this solution is diluted to 500mL, the concentration of the diluted acid will be
The density of a solution prepared by dissolving 120g of urea (mol. mass = 60u) in 1000g of water is 1.15g/mL. The molarity of this solution is
What will be the molality of a solution of glucose in water which is 10% w/W?
The molality of 648 g of pure water is
What is the mass of urea required for making 2.5 kg of 0.25 molal aqueous solution?
Concentration terms like mass percentage, ppm, mole fraction and molality do not depend on temperature. However, molarity is a function of temperature because
|
108 videos|287 docs|123 tests
|


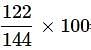 = 84.72%
= 84.72%