Test: Isolation of Elements - 2 (Old NCERT) - NEET MCQ
30 Questions MCQ Test Inorganic Chemistry for NEET - Test: Isolation of Elements - 2 (Old NCERT)
Formation of metallic copper from the sulphide ore in the commercial thermo-metallurgical process essentially involves which one of the following reaction :
Ag2S + NaCN + Zn →Ag
This method of extraction of Ag by complex formation and then its displacement is called :
| 1 Crore+ students have signed up on EduRev. Have you? Download the App |
Calcination is the process of heating the ore :
Which of the following does not contain Mg :
Match the method of concentration of the ore in column I with the ore in column II and select the correct alternate:
Bessemerization is carried out for
(I) Fe
(II) Cu
(III) Al
(IV) silver
Which of the following is not used for obtaining Ag
Which one of the following is not a method of concentration of ore?
In which of the following isolations no reducing agent is required :
Chemical leaching is useful in the concentration of :
The element which could be extracted by electrolytic reduction of its oxide dissolved in a high temperature melt is :
Consider the following statements :
Roasting is carried out to :
(i) convert sulphide to oxide and sulphate (ii) remove water of hydration
(iii) melt the ore (iv) remove arsenic and sulphur impurities
Of these statements :
Iron obtained from blast furance is :
Which one of the following statements is not correct :
Which of the following is not an ore :
In the extraction of nickel by Mond process, the metal is obtained by :
Froath floatation process is based on:
When ZnS and PbS minerals are present together, then NaCN is added to separate them in the froth floatation process as a depressant, because
When copper is purified by electrorefining process, noble metals like Ag and Au are found in
Formation of Ni(CO)4 and subsequent its decomposition into Ni and CO (recycled) makes basis of Mond's process
Ni + 4CO 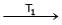 Ni (CO)4
Ni (CO)4 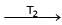 Ni + 4CO
Ni + 4CO
T1 and T2 are :
Match column–I (process) with column–II (electrolyte) :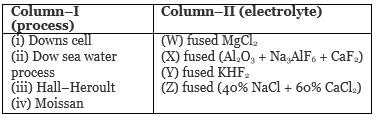
Choose the correct alternate.
(I) FeCr2O4 + NaOH + air → (A) + Fe2O3
(II) (A) + (B) → Na2Cr2O7
(III) Na2Cr2O7 + X Cr2O3
(IV) Cr2O3 + Y Cr
Q.
Compounds (A) and (B) are :
(I) FeCr2O4 + NaOH + air → (A) + Fe2O3
(II) (A) + (B) → Na2Cr2O7
(III) Na2Cr2O7 + X Cr2O3
(IV) Cr2O3 + Y Cr
Q.
(X) and (Y) are :
(I) FeCr2O4 + NaOH + air → (A) + Fe2O3
(II) (A) + (B) → Na2Cr2O7
(III) Na2Cr2O7 + X Cr2O3
(IV) Cr2O3 + Y Cr
Q.
Na2CrO4 and Fe2O3 are separated by :
(I) FeCr2O4 + NaOH + air → (A) + Fe2O3
(II) (A) + (B) → Na2Cr2O7
(III) Na2Cr2O7 + X Cr2O3
(IV) Cr2O3 + Y Cr
Q.
High temperature (> 1000ºC) electrolytic reduction is necessary for isolating :
In froth-floatation process, palm oil functions as :
Collector are the substances which help in attachement of an ore particle to air bubble in froth. A popular collector used industrially is :
|
74 videos|106 docs|111 tests
|
|
74 videos|106 docs|111 tests
|


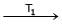 Ni (CO)4
Ni (CO)4 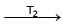 Ni + 4CO
Ni + 4CO














